Tracking chondrocyte-to-fibroblast transformation via changes in cell electrophysiology
- PMID: 40335587
- PMCID: PMC12059058
- DOI: 10.1038/s41598-025-98958-1
Tracking chondrocyte-to-fibroblast transformation via changes in cell electrophysiology
Abstract
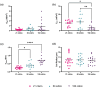
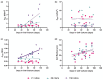
Cultured chondrocytes have potential for cartilage regeneration to treat degenerative diseases. However, when explanted chondrocytes are cultured in monolayer, they are known to dedifferentiate over time, adopting a more fibroblastic phenotype. This greatly impacts both research and potential clinical applications; cell-based cartilage repair therapies require significant in vitro expansion to obtain sufficient chondrocyte numbers for reimplantation, as chondrocytes adopt a more fibroblastic phenotype causing up to 70% of patients to develop fibrocartilaginous fill. We used dielectrophoresis (DEP) to observe changes in the electrophysiological properties of primary bovine chondrocytes over time. Using a multi-conductivity approach, we demonstrate that monitoring the cytoplasmic conductivity is a reliable method of observing cell changes over 100 days in culture. Results show statistically significant changes in both membrane capacitance (p = 0.0039) and cytoplasm conductivity (p = < 0.0001) when tested at multiple conductivities. Analysis of cytoplasmic vs. medium conductivity allowed simple tracking of chondrocyte membrane potential, which exhibited transitions between three stable values of Vm which correspond to patch-clamp-derived literature values as they transition from chondrocytes (- 13 to - 18 mV) to proliferating fibroblasts (- 32 to - 43 mV) and ultimately to non-proliferating fibroblasts (- 55 to - 71 mV), transitions occurring around days 40 and 80 respectively.
Keywords: Biomarker; Dedifferentiation; Dielectrophoresis; Electrome; Membrane potential.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: Author MPH is co-inventor of the 3DEP instrument used in this work and a director of the company that manufactures it. The remaining authors declare no other conflicts of interest.
Figures


Similar articles
-
Centrifugation assay for measuring adhesion of serially passaged bovine chondrocytes to polystyrene surfaces.Tissue Eng Part C Methods. 2012 Jul;18(7):537-44. doi: 10.1089/ten.TEC.2011.0043. Epub 2012 Feb 24. Tissue Eng Part C Methods. 2012. PMID: 22235797
-
P38 mitogen-activated protein kinase promotes dedifferentiation of primary articular chondrocytes in monolayer culture.J Cell Mol Med. 2013 Apr;17(4):508-17. doi: 10.1111/jcmm.12034. Epub 2013 Mar 11. J Cell Mol Med. 2013. PMID: 23480786 Free PMC article.
-
Maintenance of cartilaginous gene expression on extracellular matrix derived from serially passaged chondrocytes during in vitro chondrocyte expansion.J Biomed Mater Res A. 2012 Mar;100(3):694-702. doi: 10.1002/jbm.a.34003. Epub 2011 Dec 30. J Biomed Mater Res A. 2012. PMID: 22213591
-
Advances in autologous chondrocyte implantation and related techniques for cartilage repair.Dan Med J. 2013 Apr;60(4):B4600. Dan Med J. 2013. PMID: 23651721 Review.
-
Feasibility of Human Platelet Lysate as an Alternative to Foetal Bovine Serum for In Vitro Expansion of Chondrocytes.Int J Mol Sci. 2021 Jan 28;22(3):1269. doi: 10.3390/ijms22031269. Int J Mol Sci. 2021. PMID: 33525349 Free PMC article. Review.
References
-
- Mao, Y. et al. Extracellular matrix derived from chondrocytes promotes rapid expansion of human primary chondrocytes in vitro with reduced dedifferentiation. Acta Biomater.85, 75–83. 10.1016/j.actbio.2018.12.006 (2019). - PubMed
-
- Layman, D. L., Sokoloff, L. & Miller, E. J. Collagen synthesis by articular in monolayer culture. Exp. Cell Res.73, 107–112. 10.1016/0014-4827(72)90107-3 (1972). - PubMed
-
- Lin, Z. et al. Gene expression profiles of human chondrocytes during passaged monolayer cultivation. J. Orthop. Res.26, 1230–1237. 10.1002/jor.20523 (2008). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

