Longitudinal stability of HyperSightTM-CBCT based radiomic features in patients with CT guided adaptive SBRT for prostate cancer
- PMID: 40335645
- PMCID: PMC12059028
- DOI: 10.1038/s41598-025-99920-x
Longitudinal stability of HyperSightTM-CBCT based radiomic features in patients with CT guided adaptive SBRT for prostate cancer
Abstract
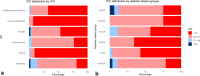
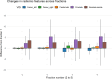
CT-guided adaptive radiotherapy (aRT) based on HyperSightTM-CBCT provides high-quality imaging, allowing quantitative radiomic feature analysis as a monitoring tool. This study comprehensively evaluates the stability of radiomic features, as potential imaging biomarkers, in pelvic structures of prostate cancer patients treated with adaptive stereotactic body radiation therapy (SBRT). Between December 2023 and July 2024, 32 patients with localized prostate cancer underwent adaptive SBRT at the Ethos® linear accelerator (Varian, Siemens Healthineers) with HyperSight-CBCT imaging. Longitudinal stability was assessed by intraclass correlation coefficient (ICC) over five fractions of aRT for target structures and non-hollow organs at risk. In pooled organs at risk, 93.0% of features showed very high stability (ICC > 0.9) compared to 67.4% in pooled target structures, indicating significantly lower stability for target structures (p = 0.00009129). Second-order features demonstrated greater stability than conventional and shape-based features (p = 0.0433, p = 0.0252). Fraction number significantly affected longitudinal prostate feature variability (p = 0.0135). This study comprehensively analyzed HyperSight-CBCT imaging to evaluate longitudinal stability of radiomic features during adaptive SBRT for prostate cancer. The trends observed will provide a framework for future CT-guided aRT studies, facilitating quantitative imaging analysis of radiological biomarkers for clinical translation and improving personalized treatment.
Keywords: Adaptive radiotherapy; Ethos®; HyperSight-CBCT; Prostate cancer; Radiomics.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: DB reports NB Capital ApS (Consultation, personal fees), PharmaMar GmbH (speaker), AstraZeneca GmbH (speaker). FG reports travel expenses, stocks and honoraria from TME Pharma AG related to this work; research grants and travel expenses from ELEKTA AB; grants, research grants, travel expenses and honoraria from Carl Zeiss Meditec AG; grants, research grants, travel expenses and honoraria from OncoMANGETx, Inc.; travel expenses and research grants from Varian Medical Systems, Inc.; travel expenses and/or honoraria from Bristol- Myers Squibb, Cureteq AG, Roche Pharma AG, MSD Sharp and Dohme GmbH, Siemens Healthineers AG, Varian Medical Systems, and AstraZeneca GmbH; non-financial support from Oncare GmbH and Opasca GmbH and patent US10857388B2 together with Carl Zeiss Meditec AG and patent EP4119191A1. JBH reports AstraZeneca speaker fees and consulting honoraria EbaMed SA. CD reports Varian research grant and travel expenses, AstraZeneca speaker fees and travel expenses, and DGVS speaker fees and travel expenses. All other authors have no competing interests to declare.
Figures




References
-
- Yan, D. et al. Adaptive radiation therapy. Phys. Med. Biol.42(1), 123–132 (1997). - PubMed
-
- Møller, D. S. et al. Adaptive radiotherapy for advanced lung cancer ensures target coverage and decreases lung dose. Radiother. Oncol.121(1), 32–38 (2016). - PubMed
-
- Christiansen, R. L. et al. Online adaptive radiotherapy potentially reduces toxicity for high-risk prostate cancer treatment. Radiother. Oncol.167, 165–171 (2022). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

