Harnessing nutrient scarcity for enhanced CAR-T-cell potency and safety in solid tumors
- PMID: 40335738
- PMCID: PMC12125372
- DOI: 10.1038/s41423-025-01290-x
Harnessing nutrient scarcity for enhanced CAR-T-cell potency and safety in solid tumors
Abstract
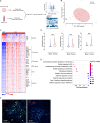
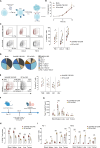
Despite significant advancements, the effectiveness of chimeric antigen receptor (CAR)-T-cell-based therapies in solid tumors remains limited. Key challenges include on-target effects, off-tumor toxicity and reduced CAR-T-cell function within the tumor microenvironment, which is often characterized by metabolic stress triggered by factors such as amino acid scarcity. Activating transcription factor-4 (ATF4) and its upstream regulator GCN2 play crucial roles in the metabolic reprogramming and functionality of CD4+ and CD8+ T cells. ATF4 can be activated by various cellular stress signals, including amino acid deprivation. While ATF4 activation may be associated with T-cell dysfunction, its role in stress adaptation presents an opportunity for therapeutic intervention-particularly in the tumor microenvironment, where T-cell exhaustion is a major challenge. In this study, we developed a strategy to harness the GCN2‒ATF4 axis in CAR-T cells. We employed an amino acid-dependent inducible promoter, which triggers ATF4-dependent gene expression to regulate CAR expression in T cells under conditions of amino acid scarcity within the tumor microenvironment. In vitro and murine xenograft models demonstrate the potential of this system to effectively restrict CAR expression to the tumor site. This targeted strategy not only enhances safety by minimizing off-tumor activity but also CAR-T-cell fitness by reducing exhaustion. By validating this pathophysiologically regulatable CAR expression system for solid tumors, our findings address key limitations of current CAR-T-cell therapies and pave the way for innovative strategies targeting solid malignancies.
Keywords: Amino acid scarcity; CAR-T; Solid tumours; Tumour microenvironment.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Benjamin Versier, Aravindhan Soundiramourty, Yves Christen, Dominique Charron, Jacques Mallet, and Che Serguera serve Asfalia Biologics. Ludmila Juricek previously served at Asfalia Biologics and currently serves for Coave Therapeutics. The authors declare that a patent application related to this work has been filed. The other authors declare that they have no conflicts of interest.
Figures





References
-
- Holstein SA, Lunning MA. CAR T-Cell Therapy in hematologic malignancies: a voyage in progress. Clin Pharmacol Ther. 2020;107:112–22. - PubMed
-
- Saleki K, Mohamadi MH, Alijanizadeh P, Rezaei N. Neurological adverse effects of chimeric antigen receptor T-cell therapy. Expert Rev Clin Immunol. 2023;19:1361–83. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

