ELF4 improves sepsis-induced myocardial injury by regulating STING signaling-mediated T cells differentiation
- PMID: 40335763
- PMCID: PMC12058945
- DOI: 10.1007/s10565-025-10029-3
ELF4 improves sepsis-induced myocardial injury by regulating STING signaling-mediated T cells differentiation
Abstract
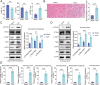
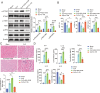
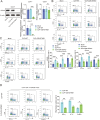
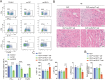
Septic cardiomyopathy (SCM) is a common complication caused by sepsis. T cells differentiation is involved in SCM progression. However, the role and underlying mechanisms of T cells-mediated immunity in SCM remain unclear. This study aimed to investigate the role of STING-mediated T cells differentiation in SCM. Cecal ligation and puncture (CLP) surgery was conducted in mice to establish SCM model. The mice were injected intraperitoneally with STING agonist ADU-S100 and C-176 after modeling. Wild type (WT) mice and CD4-STING-/- mice were employed. Besides, overexpressing vectors of ELF4 (oe-ELF4), short hairpin RNA targeting ELF4 (sh-ELF4) were transfected into 293T cells. STING signaling was found to be activated in sepsis-induced myocardial immune injury in mice. The administration of ADU-S100 exacerbated myocardial injury and inflammation, while C-176 alleviated these effects. Additionally, STING activation influenced T cells differentiation, with an increase in Th1 and Th17 cells and a decrease in Treg cells. Conditional knockout of STING in CD4+ T cells reduced Th1 and Th17 populations and improved myocardial function and histology. Furthermore, ELF4 was found to inhibit STING activation, reducing T cells differentiation into pro-inflammatory subsets. Overexpression of ELF4 in CD4+ T cells ameliorated myocardial damage and improved cardiac function in CLP mice, suggesting that the ELF4-STING signaling axis plays a protective role in sepsis-induced myocardial injury by regulating T cells differentiation.
Keywords: ELF4; STING; Septic cardiomyopathy; T cells differentiation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The animal use protocol listed has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of The Second Xiangya Hospital, Central South University (No. 2021229). Conflict of interest: The authors declare no competing interests.
Figures




Similar articles
-
CD40L-Activated DC Promotes Th17 Differentiation and Inhibits Th2 Differentiation in Sepsis-Induced Lung Injury via cGAS-STING Signaling.Biochem Genet. 2025 Jun;63(3):2455-2469. doi: 10.1007/s10528-024-10835-0. Epub 2024 May 27. Biochem Genet. 2025. PMID: 38802692
-
The transcription factor E74-like factor 4 suppresses differentiation of proliferating CD4+ T cells to the Th17 lineage.J Immunol. 2014 Jan 1;192(1):178-88. doi: 10.4049/jimmunol.1301372. Epub 2013 Nov 20. J Immunol. 2014. PMID: 24259505 Free PMC article.
-
STING-mediated intestinal barrier dysfunction contributes to lethal sepsis.EBioMedicine. 2019 Mar;41:497-508. doi: 10.1016/j.ebiom.2019.02.055. Epub 2019 Mar 14. EBioMedicine. 2019. PMID: 30878597 Free PMC article.
-
Pretreatment with interleukin-15 attenuates inflammation and apoptosis by inhibiting NF-κB signaling in sepsis-induced myocardial dysfunction.Eur J Histochem. 2024 Apr 29;68(2):4019. doi: 10.4081/ejh.2024.4019. Eur J Histochem. 2024. PMID: 38686889 Free PMC article.
-
Ferroptosis targeting offers a therapeutic target for septic cardiomyopathy.Tissue Cell. 2025 Aug;95:102930. doi: 10.1016/j.tice.2025.102930. Epub 2025 Apr 25. Tissue Cell. 2025. PMID: 40288080 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

