COX-2 inhibition as a therapeutic strategy for bone loss in Staphylococcus aureus osteomyelitis
- PMID: 40335904
- PMCID: PMC12057237
- DOI: 10.1186/s10020-025-01202-9
COX-2 inhibition as a therapeutic strategy for bone loss in Staphylococcus aureus osteomyelitis
Abstract
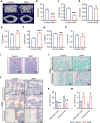
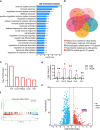
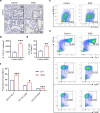
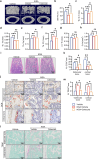
Bone loss in Staphylococcus aureus (S. aureus) osteomyelitis poses a serious challenge to orthopedic treatment, but the underlying mechanism of systemic osteoporosis caused by chronic infection is not completely clear. In this study, γ-irradiation-killed S. aureus (IKSA) was applied to simulate the inflammation and explore the mechanism of systemic bone loss caused by it. In this study, we found that the systemic application of IKSA caused bone loss in mice through increasing osteoclasts and decreasing osteoblasts. An immune response profile with up-regulated COX-2 is identified based on our transcriptional data from IKSA mice bone marrow cells. COX-2 expression is widely up-regulated in bone marrow immune cells, such as myeloid-derived suppressor cells (MDSCs), neutrophils and macrophages in the IKSA-treated mice. Mechanistically, COX-2 stimulated the increasing proportion of MDSCs and neutrophils and the inflammatory response of the bone marrow immune cells, that may regulate bone metabolism. Importantly, COX-2 inhibitor, celecoxib could rescue the bone loss induced by IKSA, which may reason from decrease of inflammatory gene expression in MDSCs, neutrophils and macrophages. Excitingly, COX-2 expression is also increased in bone marrow from mice and patients with S. aureus osteomyelitis. These findings suggested a therapeutic potential for inhibiting COX-2 in combating bone loss in S. aureus osteomyelitis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The research was ethically sanctioned by the Ethics Committee at Nanfang Hospital. Each participant in this research provided informed consent before taking part. (Approval Number: NFEC-2020-074). All procedures involving animals received approval from the Institutional Animal Care and Use Committee of Nanfang Hospital and were conducted according to the guidelines of the National Regulations on the Management of Laboratory Animals. (Approval Number: NFYY-2022-01037). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
G-CSF partially mediates bone loss induced by Staphylococcus aureus infection in mice.Clin Sci (Lond). 2019 Jun 19;133(12):1297-1308. doi: 10.1042/CS20181001. Print 2019 Jun 28. Clin Sci (Lond). 2019. PMID: 31175224
-
MyD88 and IL-1R signaling drive antibacterial immunity and osteoclast-driven bone loss during Staphylococcus aureus osteomyelitis.PLoS Pathog. 2019 Apr 12;15(4):e1007744. doi: 10.1371/journal.ppat.1007744. eCollection 2019 Apr. PLoS Pathog. 2019. PMID: 30978245 Free PMC article.
-
Naringin exerts antibacterial and anti-inflammatory effects on mice with Staphylococcus aureus-induced osteomyelitis.J Biochem Mol Toxicol. 2024 Jul;38(7):e23753. doi: 10.1002/jbt.23753. J Biochem Mol Toxicol. 2024. PMID: 38923626
-
Intracellular Staphylococcus aureus in osteoblasts and osteocytes and its impact on bone homeostasis during osteomyelitis.Bone. 2025 Sep;198:117536. doi: 10.1016/j.bone.2025.117536. Epub 2025 May 18. Bone. 2025. PMID: 40393553 Review.
-
Staphylococcus aureus vs. Osteoblast: Relationship and Consequences in Osteomyelitis.Front Cell Infect Microbiol. 2015 Nov 26;5:85. doi: 10.3389/fcimb.2015.00085. eCollection 2015. Front Cell Infect Microbiol. 2015. PMID: 26636047 Free PMC article. Review.
Cited by
-
Mitochondrial metabolic regulation of macrophage polarization in osteomyelitis and other orthopedic disorders: mechanisms and therapeutic opportunities.Front Cell Dev Biol. 2025 Jun 13;13:1604320. doi: 10.3389/fcell.2025.1604320. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40584964 Free PMC article. Review.
References
-
- Arfeen M, Srivastava A, Srivastava N, Khan RA, Almahmoud SA, Mohammed HA. Design, classification, and adverse effects of NSAIDs: a review on recent advancements. Bioorg Med Chem. 2024;112: 117899. 10.1016/j.bmc.2024.117899. - PubMed
MeSH terms
Substances
Grants and funding
- 2022A0007/the President Foundation of Nanfang Hospital, Southern Medical University
- 202412121060/College Students' Innovative Entrepreneurial Training Plan Program
- 82302791/the National Natural Science Foundation Youth Project
- 202201011339/the Science and Technology Project of Guangzhou
- 2022YFC2504305/the National Key Research and Development Program of China
LinkOut - more resources
Full Text Sources
Medical
Research Materials

