Soil microorganism colonization influenced the growth and secondary metabolite accumulation of Bletilla striata (Thunb.) Rchb. F
- PMID: 40335962
- PMCID: PMC12060540
- DOI: 10.1186/s12866-025-03960-2
Soil microorganism colonization influenced the growth and secondary metabolite accumulation of Bletilla striata (Thunb.) Rchb. F
Abstract
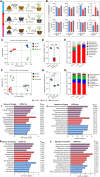
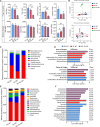
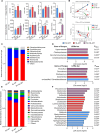
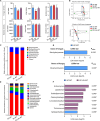
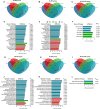
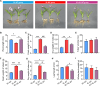
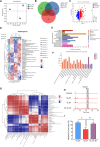
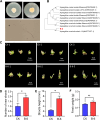
Bletilla striata (Thunb.) Rchb. F., a perennial herbaceous plant renowned for its medicinal properties, exhibits growth and secondary metabolite production that are significantly influenced by soil microorganisms. Exploring how soil microorganisms influence the growth and secondary metabolites of B. striata, we cultivated sterile seedlings in radiation-sterilized soil inoculated with microbiota from either sandy clay or sandy loam soils. Following a two-year growth period, we employed 16S and ITS Illumina sequencing to analyze the bacterial and fungal communities colonizing the rhizosphere soil, roots, tubers, and leaves of B. striata. Concurrently, we assessed the growth indices of the plants and utilized UHPLC-MS/MS to quantify the metabolites in the tubers, with a particular focus on the index component militarine and single bacteria were isolated for verification. Our findings revealed significant variations in the metabolite profiles and growth of B. striata across different soil microbial treatments. Specifically, sandy loam microorganisms were found to enhance plant growth, whereas sandy clay microorganisms increased the concentration of secondary metabolites. We identified specific microbes predominantly in loam soil that colonized roots and promoted growth (e.g., Entrophospora, Aspergillus, Fusarium). Similarly, certain microbes in loam soil colonized tubers and enhanced their growth (e.g., Sphingomonas, Hyphomicrobium). Additionally, microbes predominantly found in sandy soil colonized tubers and stimulated the synthesis of secondary metabolites (e.g., Myrmecridium, Apiotrichum montevideense). Notably, Aspergillus versicolor (B-6), isolated from the rhizosphere soil of B. striata after the introduction of sandy loam microorganisms, demonstrated a growth-promoting effect on sterile seedlings upon inoculation. This study elucidates the role of soil microorganisms in colonizing various regions of B. striata, thereby modulating its growth and secondary metabolite production. These insights have significant implications for optimizing the yield and quality of B. striata in both medicinal and agricultural applications.
Keywords: Bletilla striata; Growth and development; Microbial colonization; Secondary metabolites; Soil microbiota.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study did not involve animal or human trials. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

