Hepatic artery infusion chemotherapy combined with camrelizumab and apatinib as conversion therapy for patients with unresectable hepatocellular carcinoma: a single-arm exploratory trial
- PMID: 40335980
- PMCID: PMC12056981
- DOI: 10.1186/s12885-025-14250-5
Hepatic artery infusion chemotherapy combined with camrelizumab and apatinib as conversion therapy for patients with unresectable hepatocellular carcinoma: a single-arm exploratory trial
Abstract
Background: The development of systemic therapy, including targeted drugs and immune checkpoint inhibitors, has significantly improved the prognosis of patients with advanced unresectable hepatocellular carcinoma (uHCC). Hepatic arterial infusion chemotherapy (HAIC) has been gradually applied to the treatment of advanced uHCC, showing good potential as conversion therapy. We aimed to investigate the efficacy and safety of HAIC combined with camrelizumab and apatinib as conversion therapy for uHCC.
Methods: This study was a single-arm exploratory trial (NCT05099848) in patients with uHCC. Eligible patients received apatinib 250 mg once daily, camrelizumab 200 mg on day 3, and HAIC with FOLFOX regimen (oxaliplatin 85 mg/m2 at hours 0-2, leucovorin 400 mg/m2 at hours 2-3, and fluorouracil 400 mg/m2 at hour 3, followed by fluorouracil 2400 mg/m2 for 46 h) on days 4-5 of each 21-day cycle for up to 8 cycles. Primary endpoints were conversion rate and margin-free (R0) resection rate.
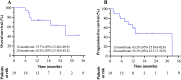
Results: Between March 2021 and July 2023, 19 patients were enrolled. Median follow-up was 14.9 months (interquartile range, 10.9-21.1). Disease became resectable in 14 (73.7%) of 19 patients; nine (47.4%) patients received R0 resection, while five (26.3%) refused surgery and opted for observation. Three (33.3%) of nine patients with surgery achieved major pathological response, including two (22.2%) with pathological complete response. Objective response and disease control rates were 47.4% (9/19) and 89.5% (17/19) per Response Evaluation Criteria In Solid Tumors (RECIST) version 1.1 and both 89.5% (17/19) per modified RECIST. Survival data were immature. Fourteen (73.7%) of 19 patients had grade 3 or higher treatment-related adverse events, with the most common being increased alanine aminotransferase or aspartate aminotransferase (seven [36.8%]) and increased lymphocyte count (six [31.6%]). No treatment-related deaths occurred.
Conclusions: The combination of HAIC, camrelizumab, and apatinib as conversion therapy shows promising clinical benefits and a manageable safety profile in patients with uHCC. Future randomized controlled trials are warranted.
Trial registration: ClinicalTrials.gov NCT05099848. Registered on October 13, 2021.
Keywords: Apatinib; Camrelizumab; Conversion therapy; Hepatic arterial infusion chemotherapy; Unresectable hepatocellular carcinoma.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All patients provided written informed consent, and the Shandong Cancer Hospital Research Institute Ethics Committee approved the protocol (No. SDZLEC2021-021–01). Consent for publication: Not applicable. Competing interests: L.Z. is on the speakers’ bureau for Bayer, MSD, AstraZeneca, Roche, BeiGene, Innovent, Junshi Biosciences and Jiangsu Hengrui Pharmaceuticals. The remaining authors declare no competing interests.
Figures



References
-
- Qin S, Chan SL, Gu S, Bai Y, Ren Z, Lin X, et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): a randomised, open-label, international phase 3 study. Lancet. 2023;402(10408):1133–46. 10.1016/S0140-6736(23)00961-3. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

