Cyclic cell-penetrating peptide-engineered ceria nanoparticles for non-invasive alleviation of ultraviolet radiation-induced cataract
- PMID: 40336002
- PMCID: PMC12060572
- DOI: 10.1186/s12951-025-03402-1
Cyclic cell-penetrating peptide-engineered ceria nanoparticles for non-invasive alleviation of ultraviolet radiation-induced cataract
Abstract
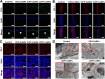
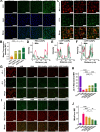
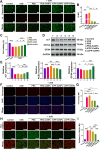
Oxidative stress, which results from the accumulation of free radicals, plays a substantial role in cataract formation. Antioxidants have shown promise in mitigating or even preventing this process. However, delivering antioxidants noninvasively to the anterior segment of the eye has been a significant challenge. In this study, we developed ceria nanoparticles modified with cyclic cell-penetrating peptides to overcome the obstruction of the dense corneal barrier on topical drug delivery. Our results demonstrated that modified ceria nanoparticles with cell-penetrating peptides (CPPs) facilitate the opening of tight junctions in human corneal epithelial cells. This characteristic considerably enhances the trans-corneal transport of nanoparticles and improves cellular uptake efficiency, while also contributing to their intracellular enrichment toward mitochondria. Further experiments confirmed that the modified ceria nanoparticles effectively counteracted ferroptosis induced by oxidative stress in lens epithelial cells both in vitro and in vivo, substantially reducing cataract formation. The successful development of ceria nanoparticles modified with cyclic cell-penetrating peptides (cCPPs) opens new avenues for research in cataract prevention and treatment. Additionally, the modified ceria nanoparticles could serve as a noninvasive drug delivery system, which holds remarkable potential for advancing drug delivery in diseases affecting the anterior segment of the eye.
Keywords: Antioxidants; Cataracts; Ceria nanoparticles; Cyclic cell-penetrating peptides; Ferroptosis; Oxidative stress.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal experiments have complied with the Association for Research in Vision and Ophthalmology Statement and the guidelines for the Animal Care and Use Committee, Zhejiang University. All animal experimental protocols were approved by the Animal Ethics Committee, the Second Affiliated Hospital, School of Medicine, Zhejiang University (Approval number: 2023–084). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




References
-
- Pesudovs K, et al. Cataract-related blindness and vision impairment in 2020 and trends over time in relation to VISION 2020: the right to sight: an analysis for the global burden of disease study. Invest Ophthalmol Vis Sci. 2021;62(8):3523.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

