Evolution of mobility, pain/discomfort, self-care, and mental health in patients with alpha-mannosidosis: an international caregiver and patient survey
- PMID: 40336024
- PMCID: PMC12057280
- DOI: 10.1186/s13023-025-03694-4
Evolution of mobility, pain/discomfort, self-care, and mental health in patients with alpha-mannosidosis: an international caregiver and patient survey
Abstract
Background: Alpha-mannosidosis is a rare recessive lysosomal storage disorder with progressive multi-systemic impacts. In the absence of standardized monitoring protocols, there is insufficient understanding of disease progression over time. This study explored the evolution of the burden of illness and quality of life (QoL) experienced by patients with alpha-mannosidosis via an international patient and caregiver-based survey. The online survey was distributed to adult patients/caregivers of patients ≥ 10 years old. It included visual analogue scales (VAS; timepoints 5 years ago and now), multiple choice, and open text questions. We report a subset of functional and QoL data: walking ability, pain/discomfort, ability to self-care, and mental health.
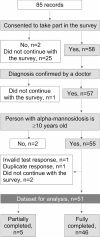
Results: Analyses include 51 responses from 18 countries: 26 patients were on velmanase alfa enzyme replacement therapy (ERT), seven had been treated with hematopoietic stem cell transplantation (HSCT) and 18 were untreated patients (UP). Over 5 years, VAS scores showed the least decline in walking ability for HSCT patients (+ 0.1 ± 1.9) compared to patients receiving ERT (+ 0.7 ± 1.2) and UP (+ 1.8 ± 2.0). A trend towards improvement in pain was only observed for those on ERT (-0.2 ± 2.0), both for pediatric and adult patients. Ability to self-care improved for patients treated with HSCT (-1.0 ± 1.8) and slightly improved with ERT (-0.3 ± 1.5) but worsened for UP (+ 0.6 ± 0.9). Similarly, a trend towards improvement in mental health scores was observed for patients on ERT (-0.4 ± 2.2).
Conclusions: Alpha-mannosidosis is associated with a substantial and progressive burden in UP, including deterioration in walking ability, pain, self-care and mental health. The survey results suggest that treatment with ERT or HSCT may slow this natural progression of alpha-mannosidosis, with these patients following a different disease trajectory to those solely receiving supportive care. This study could inform the natural pathway of alpha-mannosidosis to recognize patients' needs, courses of care, and the design of interventional studies.
Keywords: Alpha-mannosidosis; Mental health; Pain; Self-care; Survey; Walking.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: RDRP conducted this market research in accordance with the British Healthcare Business Intelligence Association’s (BHBIA) Legal & Ethical Guidelines for Market Research. In line with these standards, this market research did not require review of a Research Ethics Committee because it falls outside the remit of the UK Research Governance Framework. The confidentiality of information gathered during the study is protected in accordance with applicable data protection legislation and BHBIA’s Legal & Ethical Guidelines. Informed consent was obtained online from all participants in their local language at the start of the online survey. Respondents to the survey had to confirm they were 18 years old or over. Those not providing consent were screened out and were not able to continue with the survey. Consent for publication: Consent was obtained from participants for the use of quotes and publication. Competing interests: KMS: PI for SPARKLE registry, consulting fees and/or honoraria/travel support from Chiesi, Sanofi Genzyme, BioMarin, and Takeda, Immedica. ST: The MPS Society has received money from Chiesi for participation and chairing of advisory boards, satellite symposiums (participation, time, travel & accommodation), Fabry matters conference, patient support services, mental health services, website re-development, consultation fees for review of lay materials. JBH: Received consulting fees and/or honoraria from Chiesi, Sanofi, Takeda, Amicus, and Immedica as well as travel support from Chiesi and Amicus. CL: Has received research grants, speakers’ honoraria and/or consultation fees from Chiesi, Amicus, Alexion, BioMarin, Sanofi, and Takeda. NMM: Has received research grants, speakers’ honoraria and/or consultation fees from Chiesi, Amicus, BioMarin, JCR, Lysogene, Sanofi, and Takeda. MJBM: PI for SPARKLE registry, no competing interests. JC: No competing interests. MLR: Has received research grants, speakers’ honoraria and/or consultation fees from Chiesi, Amicus, Sanofi, and Takeda. AB: Has received consulting fees and/or honoraria/travel support from BioMarin, Chiesi Farmaceutici S.p.A., Sanofi Genzyme, and Takeda. MM: PI for SPARKLE registry, consulting fees and/or honoraria/travel support from Chiesi, BioMarin, and Takeda. AML: Has received hospital grants as a PI for studies from Chiesi Farmaceutici S.p.A. as well as consulting fees and/or honoraria/travel support from Alexion, BioMarin, Chiesi Farmaceutici S.p.A., Sanofi Genzyme, and Shire. VP: PI for SPARKLE registry, speakers’ honoraria/travel support and/or consulting fees from BioMarin, Chiesi, Sanofi Genzyme, Shire/Takeda. AB: Former employee of Chiesi Farmaceutici S.p.A. during the time of this study. FD: Employee of Chiesi Farmaceutici S.p.A. HMM: Employee of Chiesi USA Inc. NG: Has received hospital grants as well as consulting fees and/or honoraria/travel support from BioMarin, Chiesi Farmaceutici S.p.A., JCR pharmaceuticals, Sanofi winthrop, Ultragenyx, and Takeda.
Figures




References
-
- OMIM®. Mannosidosis, Alpha B, Lysosomal, MANSA. Available online: https://www.omim.org/entry/248500. (Accessed: 6 February 2024).
-
- Malm D, Nilssen Ø. Alpha-mannosidosis. Orphanet encyclopedia. Available online: https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=en%26Expert=61. (Accessed: 2 January 2024).
-
- Ficicioglu C, Stepien KM. Alpha-Mannosidosis. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, et al. editors. GeneReviews(®). Seattle (WA): University of Washington, Seattle. Copyright © 1993–2024, University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved.; 2001. Oct 11 [updated 2024 Jun 13].
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

