Advances in cancer immunotherapy: historical perspectives, current developments, and future directions
- PMID: 40336045
- PMCID: PMC12057291
- DOI: 10.1186/s12943-025-02305-x
Advances in cancer immunotherapy: historical perspectives, current developments, and future directions
Abstract
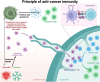
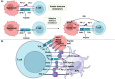
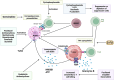
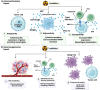
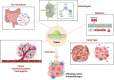
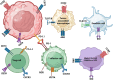
Cancer immunotherapy, encompassing both experimental and standard-of-care therapies, has emerged as a promising approach to harnessing the immune system for tumor suppression. Experimental strategies, including novel immunotherapies and preclinical models, are actively being explored, while established treatments, such as immune checkpoint inhibitors (ICIs), are widely implemented in clinical settings. This comprehensive review examines the historical evolution, underlying mechanisms, and diverse strategies of cancer immunotherapy, highlighting both its clinical applications and ongoing preclinical advancements. The review delves into the essential components of anticancer immunity, including dendritic cell activation, T cell priming, and immune surveillance, while addressing the challenges posed by immune evasion mechanisms. Key immunotherapeutic strategies, such as cancer vaccines, oncolytic viruses, adoptive cell transfer, and ICIs, are discussed in detail. Additionally, the role of nanotechnology, cytokines, chemokines, and adjuvants in enhancing the precision and efficacy of immunotherapies were explored. Combination therapies, particularly those integrating immunotherapy with radiotherapy or chemotherapy, exhibit synergistic potential but necessitate careful management to reduce side effects. Emerging factors influencing immunotherapy outcomes, including tumor heterogeneity, gut microbiota composition, and genomic and epigenetic modifications, are also examined. Furthermore, the molecular mechanisms underlying immune evasion and therapeutic resistance are analyzed, with a focus on the contributions of noncoding RNAs and epigenetic alterations, along with innovative intervention strategies. This review emphasizes recent preclinical and clinical advancements, with particular attention to biomarker-driven approaches aimed at optimizing patient prognosis. Challenges such as immunotherapy-related toxicity, limited efficacy in solid tumors, and production constraints are highlighted as critical areas for future research. Advancements in personalized therapies and novel delivery systems are proposed as avenues to enhance treatment effectiveness and accessibility. By incorporating insights from multiple disciplines, this review aims to deepen the understanding and application of cancer immunotherapy, ultimately fostering more effective and widely accessible therapeutic solutions.
Keywords: Biomarker; Cancer immunotherapy; Clinical and pre-clinical; Immune evasion; Tumor microenvironment remodeling.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- Dillman RO. Cancer Immunother. Cancer Biother Radiopharm. 2011;26(1):1–64. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

