Synergistic benefit of thiazolidinedione and sodium-glucose cotransporter 2 inhibitor for metabolic dysfunction-associated steatotic liver disease in type 2 diabetes: a 24-week, open-label, randomized controlled trial
- PMID: 40336058
- PMCID: PMC12060414
- DOI: 10.1186/s12916-025-04017-x
Synergistic benefit of thiazolidinedione and sodium-glucose cotransporter 2 inhibitor for metabolic dysfunction-associated steatotic liver disease in type 2 diabetes: a 24-week, open-label, randomized controlled trial
Abstract
Background: The close interplay between metabolic dysfunction-associated steatotic liver disease (MASLD) and type 2 diabetes supports the need to identify beneficial combination therapies of antidiabetic medications targeted for the treatment of MASLD. This study aimed to investigate the complementary effects of combination therapy with pioglitazone (PIO) and empagliflozin (EMPA) on MASLD in individuals with type 2 diabetes.
Methods: In a randomized, open-label trial, 50 participants with type 2 diabetes and MASLD were assigned 1:1:1 to receive PIO 15 mg, EMPA 10 mg, or a combination (PIO 15 mg plus EMPA 10 mg) daily for 24 weeks. Liver fat fraction and stiffness were evaluated using magnetic resonance imaging-proton density fat fraction (MRI-PDFF) and magnetic resonance elastography (MRE), respectively.
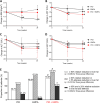
Results: Combination therapy resulted in the largest reduction in liver fat and stiffness among treatment groups. Participants experiencing a relative reduction ≥ 30% or an absolute reduction ≥ 5% in liver fat were the most prevalent in the combination group (100.0% vs. 57.1% in PIO and 87.5% in EMPA, p = 0.010). In addition, the combination group showed the highest proportion of individuals with a relative reduction ≥ 30% in liver fat and ≥ 20% in liver stiffness than the monotherapy groups (50.0% vs. 21.4% in PIO and 6.3% in EMPA, p = 0.029). Combination therapy did not induce the changes in subcutaneous fat deposition observed in the monotherapy groups, but it did show the most substantial reduction in visceral fat, concurrently showing the largest increase in adiponectin level across the three groups (p = 0.036).
Conclusions: Combination therapy of PIO with EMPA showed synergistic benefits for MASLD in individuals with type 2 diabetes, compensating for the inadequate or unfavorable effects of monotherapies; ClincialTrials.gov number, NCT03646292.
Trial registration: The trial was registered at ClinicalTrials.gov (registration number: NCT03646292).
Keywords: Metabolic dysfunction-associated steatotic liver disease; Sodium-glucose cotransporter 2 inhibitor; Thiazolidinedione; Type 2 diabetes.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All participants provided written informed consent and the Ethics Committee of the Yonsei University College of Medicine approved this study (4-2018-0655), which conforms to the ethical principles of the 1975 Declaration of Helsinki. Consent for publication: Not applicable. Competing interests: M.L. has received lecture honoraria from JW Pharmaceutical Corporation, Boryung Corporation, Eli Lilly and Company, Merck Sharp & Dohme, HK inno.N, Servier Korea, Handok Inc., Daewoong Pharmaceutical, KUKJE PHARM CO.,LTD, and GC Biopharma Corporation. All other authors have no conflicts to disclose; no other relationships or activities that could appear to have influenced the submitted work.
Figures

References
-
- Kokkorakis M, Muzurovic E, Volcansek S, Chakhtoura M, Hill MA, Mikhailidis DP, Mantzoros CS. Steatotic Liver Disease: Pathophysiology and Emerging Pharmacotherapies. Pharmacol Rev. 2024;76(3):454–99. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

