Invasive laser acupuncture targeting muscle: a novel approach to protect dopaminergic neurons and reduce neuroinflammation in a brain of Parkinson's disease model
- PMID: 40336061
- PMCID: PMC12057028
- DOI: 10.1186/s13020-025-01104-2
Invasive laser acupuncture targeting muscle: a novel approach to protect dopaminergic neurons and reduce neuroinflammation in a brain of Parkinson's disease model
Abstract
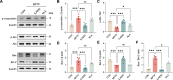
Parkinson's disease (PD) affects 1-2% of the global population and presents significant therapeutic challenges. Due to the limitations of existing treatments, there is a pressing need for alternative approaches. This study investigated the effects of invasive laser acupuncture (ILA), which combines acupuncture and photobiomodulation. In this method, optical fibers are inserted into the muscle layers of the acupoint to enhance therapeutic outcomes. Mice with MPTP-induced PD were treated with ILA at 830 nm or 650 nm. Protective effects of nigrostriatal dopaminergic neurons and fibers were assessed by examining TH immunoreactivity in the brain. Neuroinflammation markers in the brain and muscle metabolomic profiles were also analyzed. Comparisons between invasive and non-invasive laser application, as well as the impact of nerve blocking with lidocaine, were also evaluated. ILA at 830 nm (ILA830) significantly improved motor performance and increased the nigrostriatal TH-positive immunoreactivities. It reduced the levels of α-synuclein, apoptotic proteins, and inflammatory cytokines, while increasing anti-inflammatory in the brain. ILA830 also decreased nigrostriatal astrocyte and microglia activation. Muscle metabolomic analysis showed distinct group clustering and significant changes in metabolites like glucose and galactose, correlating with improved motor functions. Invasive laser treatment was more effective than non-invasive, and lidocaine pre-treatment did not block its effects. ILA at 830 nm effectively ameliorates PD symptoms by protecting dopaminergic neurons, and reducing neuroinflammation in the brain. Muscle metabolomic changes by ILA830, such as increased glucose and galactose, correlate with motor improvement. This approach offers a promising strategy for PD treatment, warranting further research to optimize its use in clinical settings.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: All experimental procedures involving animals were conducted in accordance with the guidelines of the National Institutes of Health (NIH) for the care and use of laboratory animals and were approved by the Institutional Animal Ethical Committee at Kyung Hee University. The ethics approval number is KHSASP-23-280.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

