Enoxaparin dosing for venous thromboembolism prophylaxis in hospitalized underweight adult patients: a retrospective cohort study
- PMID: 40336070
- PMCID: PMC12060466
- DOI: 10.1186/s12959-025-00716-w
Enoxaparin dosing for venous thromboembolism prophylaxis in hospitalized underweight adult patients: a retrospective cohort study
Abstract
Background: Enoxaparin is commonly used for venous thromboembolism (VTE) prophylaxis in adult hospitalized patients. Although anti-Xa levels are inversely related to body weight, limited studies evaluated clinical outcomes of dose reduction in the underweight population.
Objective: To compare the incidence of bleeding and VTE in underweight patients receiving reduced doses of enoxaparin (< 40 mg daily) versus the standard dose (40 mg daily) for VTE prophylaxis.
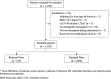
Methods: This was a multicentre retrospective cohort study at Hamad Medical Corporation in Qatar. We included hospitalized patients with a total body weight ≤ 57 kg or body mass index (BMI) ≤ 18.5 kg/m2 who received prophylactic enoxaparin for at least 48 h. The outcomes were bleeding, VTE, and composite unfavourable outcome (bleeding or VTE). Inverse-probability-of-treatment weighting (IPTW) was used to adjust for confounding.
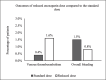
Results: We identified 1,130 eligible patients, of whom 124 patients (11%) received the reduced dose, and 1,006 patients (89%) received the standard dose. Bleeding occurred in one patient (0.8%) of the reduced dose group compared to 15 patients (1.5%) in the standard dose group (p > 0.99), VTE occurred in two patients (1.6%) in the reduced dose group compared to four patients (0.4%) in the standard dose group (p = 0.13). In the IPTW analysis, there was no significant difference in overall bleeding (odds ratio (OR) 1.4, 95% CI 0.18-10.75, p = 0.74), VTE (OR 0.3, 95% CI 0.05-1.81, p = 0.19), or the composite unfavourable outcome (OR 0.74, 95% CI 0.2-2.75, p = 0.66).
Conclusion: There is no significant difference in the incidence of bleeding or VTE between the reduced dose and the standard dose of enoxaparin for VTE prophylaxis in underweight adult patients. Due to the low event rates in both groups, larger studies are required to delineate any differences between the two dosing strategies.
Keywords: Body mass index; Deep vein thrombosis; Dose; Enoxaparin; Prophylaxis; Venous thromboembolism; Weight.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Hamad Medical Corporation Medical Research Centre approved this research (MRC- 01- 22–477). Medical Research Centre waived the informed consent due to the retrospective nature of the study. After data extraction and validation, we replaced patient identifiers with unique study codes to protect patients’ privacy. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- Enoxaparin. Lexi-Drugs. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc.; 2022. Available from: http://online.lexi.com/lco/action/home. Cited 2022 Jul 24.
LinkOut - more resources
Full Text Sources

