Lipid liquid-crystalline nanoparticles as a suitable platform for accommodating sensitive membrane proteins: monitoring the activity of HMG-CoA reductase
- PMID: 40336087
- PMCID: PMC12057187
- DOI: 10.1186/s12951-025-03370-6
Lipid liquid-crystalline nanoparticles as a suitable platform for accommodating sensitive membrane proteins: monitoring the activity of HMG-CoA reductase
Abstract
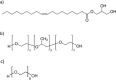
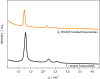
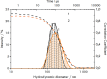
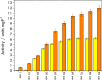
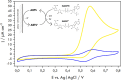
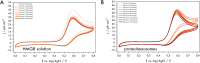
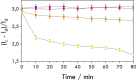
Biological molecules such as integral membrane proteins, peptides, and nucleic acids that are not soluble or sufficiently stable in aqueous solutions can be stabilized through encapsulation in lipid nanoparticles. Discovering the potential of lipid liquid-crystalline nanoparticles opens up exciting possibilities for housing sensitive membrane proteins. Lipid mesophases provide an environment that protects the cargo, usually a drug, from rapid clearance or degradation. This study employed the mentioned platform to stabilize a different cargo-an essential transmembrane enzyme, HMG-CoA reductase (HMGR). The nanostructured lipid liquid-crystalline (LLC) nanoparticles known as hexosomes are selected as a convenient nanocontainer for the redox-active protein for real-time monitoring of its functions in the bulk of the solution and point to the applicability of the proposed platform in the evaluation of therapeutic functions of the protein by standard physicochemical methods. Instead of using detergents, which usually affect the functions and stability of sensitive membrane proteins, we provide a suitable environment, protecting them in the bulk of the solution against other present species, e.g., toxic compounds or degrading proteins. The objective was to optimize the composition and structure of the lipid nanoparticles to meet the needs of such sensitive and flexible membrane proteins as HMGR and compare the functioning of the encapsulated enzyme with that of the same protein free in the aqueous solution. The catalytic reaction of HMGR involves the 4-electron reduction of HMG-CoA to mevalonate and CoA while simultaneously oxidizing NADPH to NADP+. Subsequently, mevalonate is transformed into cholesterol. The hexosomes we selected as lipid nano-containers were composed of monoolein, 1-oleoyl-rac-glycerol (GMO), Pluronic® F127, and poly(ethylene glycol) (PEG). These specific structural characteristics of the lipid nanoparticles were found optimal for enhancing the stability of HMGR. We characterized these hexosomes using dynamic light scattering (DLS), small-angle X-ray scattering (SAXS), and cryogenic electron microscopy (Cryo-TEM) methods, both with and without the encapsulated protein. In our innovative approach, the enzyme activity was assessed by monitoring changes in NADPH concentration outside the nanocarrier. We tracked fluctuations in NADPH levels during the catalytic reaction using two independent methods: UV-Vis spectrophotometry and cyclic voltammetry. Significantly, we could demonstrate the inhibition of the nano-encapsulated enzyme by fluvastatin, an enzyme inhibitor and cholesterol-lowering drug. This paves the way for the discovery of new enzymatic inhibitors and activators as therapeutic agents controlling the activity of membrane proteins, thereby inspiring future cholesterol-lowering therapies in our case and, in general, further research and potential new treatments.
Keywords: HMG-CoA reductase; NADPH; Bioelectrochemistry; Cyclic voltammetry; Hexosome; Lipid nanoparticles; Transmembrane enzyme.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: All authors read and approve the final manuscript. Competing interests: The authors declare no competing interests.
Figures
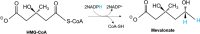



References
-
- Akinc A, Maier MA, Manoharan M, Fitzgerald K, Jayaraman M, Barros S, Ansell S, Du X, Hope MJ, Madden TD, Mui BL, Semple SC, Tam YK, Ciufolini M, Witzigmann D, Kulkarni JA, van der Meel R, Cullis PR. The onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat Nanotechnol. 2019;14(12):1084–7. 10.1038/s41565-019-0591-y. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

