Intratumor HIF-1α modulates production of a cachectic ligand to cause host wasting
- PMID: 40336592
- PMCID: PMC12056967
- DOI: 10.1016/j.cellin.2025.100247
Intratumor HIF-1α modulates production of a cachectic ligand to cause host wasting
Abstract
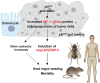
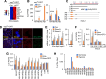
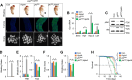
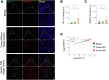
Tumor-host interactions play critical roles in cancer-associated cachexia. Previous studies have identified several cachectic proteins secreted by tumors that impair metabolic homeostasis in multiple organs, leading to host wasting. The molecular mechanisms by which malignant tumors regulate the production or secretion of these cachectic proteins, however, still remain largely unknown. In this study, we used different Drosophila cachexia models to investigate how malignant tumors regulate biosynthesis of ImpL2, a conserved cachectic protein that inhibits systemic insulin/IGF signaling and suppresses anabolism of host organs. Through bioinformatic and biochemical analysis, we found that hypoxia-inducible factor HIF-1α/Sima directly binds to the promoter region of ImpL2 gene for the first time, promoting its transcription in both tumors and non-tumor cells. Interestingly, expressing HphA to moderately suppress HIF-1α/Sima activity in adult yki 3SA gut tumors or larval scrib 1 Ras V12 disc tumors sufficiently decreased ImpL2 expression and improved organ wasting, without affecting tumor growth. We further revealed conserved regulatory mechanisms conserved across species, as intratumor HIF-1α enhances the production of IGFBP-5, a mammalian homolog of fly ImpL2, contributing to organ wasting in both tumor-bearing mice and patients. Therefore, our study provides novel insights into the mechanisms by which tumors regulate production of cachectic ligands and the pathogenesis of cancer-induced cachexia.
© 2025 Published by Elsevier B.V. on behalf of Wuhan University.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Argiles J.M., Lopez-Soriano F.J., Stemmler B., Busquets S. Cancer-associated cachexia - understanding the tumour macroenvironment and microenvironment to improve management. Nature Reviews Clinical Oncology. 2023;20:250–264. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

