Structural-Functional Correlation in Non-Arteritic Acute Ischemic Optic Neuropathy
- PMID: 40336777
- PMCID: PMC12057633
- DOI: 10.2147/EB.S512882
Structural-Functional Correlation in Non-Arteritic Acute Ischemic Optic Neuropathy
Abstract
Purpose: This study investigated the relationships between structural and functional parameters in non-arteritic ischemic optic neuropathy (NAION).
Methods: This retrospective study enrolled 29 patients (58.2 ± 10.4 years old) with unilateral NAION. During the acute phase, we performed comprehensive evaluations including best-corrected visual acuity (BCVA), optical coherence tomography (OCT), optical coherence tomography angiography (OCTA), visual fields (VF), visual evoked potentials (VEP), electroretinography (ERG), and multifocal ERG (mf-ERG). At three months post-presentation, patients underwent follow-up assessments comprising visual acuity testing, perimetry, and advanced retinal imaging.
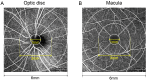
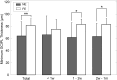
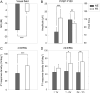
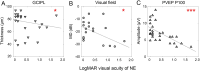
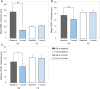
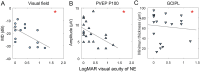
Results: During the acute phase, affected eyes demonstrated increased mean retinal nerve fiber layer (RNFL) thickness, while ganglion cell-inner plexiform layer (GCIPL) thickness decreased. Both visual fields mean deviation (MD) and VEP P100 amplitude were reduced, accompanied by prolonged peak latency. We also observed decreased P1 response density in mf-ERG. Analysis revealed significant direct correlations between GCIPL parameters and electrophysiological measurements, particularly VEP P100 amplitude and mf-ERG P1 response density. Mean GCIPL thickness, VF MD, and VEP P100 amplitude showed negative correlations with baseline logMAR VA. Baseline VF MD, VEP P100 amplitude, and minimum GCIPL thickness showed negative correlations with logMAR VA at 3-month follow-up.
Conclusion: Retinal ganglion cell layer thickness serves as a valuable indicator to objective evaluate optic nerve function in acute NAION patients. Decreases in both VEP amplitude and mf-ERG response density showed significant correlations with retinal ganglion cell layer thickness. Baseline visual field performance, VEP measurements, and minimum GCIPL thickness exhibited negative correlations visual acuity at 3-month follow-up.
Trial registration: Clinical Research Ethics Committee of Xi'an People's Hospital (NO. 20220018). Registered 27 September 2022-Retrospectively registered, https://www.medicalresearch.org.cn/. Informed consent was obtained from each participant.
Keywords: OCT; OCTA; ischemic optic neuropathy; visual electrophysiology; visual field.
© 2025 Wei et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures




References
LinkOut - more resources
Full Text Sources

