Prehospital application of remote ischaemic perconditioning in acute ischaemic stroke patients in Catalonia: the REMOTE-CAT clinical trial
- PMID: 40337071
- PMCID: PMC12056375
- DOI: 10.1016/j.eclinm.2025.103208
Prehospital application of remote ischaemic perconditioning in acute ischaemic stroke patients in Catalonia: the REMOTE-CAT clinical trial
Abstract
Background: Acute ischaemic stroke (IS) remains one of the leading causes of morbidity and mortality worldwide. Remote ischaemic perconditioning (RIperC) is a neuroprotective treatment with promising preclinical results, acting through humoral and neural mechanisms. This trial aimed to evaluate the clinical benefits of prehospital-initiated RIperC in acute IS patients.
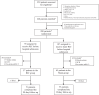
Methods: REMOTE-CAT was a multicentre, randomised, double-blind, sham-controlled trial across four Catalonian stroke centres. Patients over 18 years with stroke symptoms under 8 h, a pre-stroke modified Rankin Scale (mRS) score <3, and motor deficits (RACE motor score ≥1) were randomised 1:1 to active RIperC or sham. RIperC was applied via an automated cuff on the unaffected arm in five 5-min inflation-deflation cycles. Investigators and participants were blinded to treatment. The primary outcome was the proportion of patients with a favourable outcome (mRS <3) at 90 days. The intention-to-treat analysis included all patients receiving at least one inflation-deflation cycle and had a final diagnosis of ischaemic stroke or transient ischaemic attack (ClinicalTrials.gov: NCT03375762).
Findings: Between August 2019 and December 2023, 350 patients were screened, with 200 randomised. After 78 exclusions (29 haemorrhagic strokes, 41 stroke mimics, and 8 patients with mRS >3), 122 patients were included in the primary analysis (RIperC group, n = 57; sham group, n = 65). The RIperC group had a higher proportion of mRS <3 at 90 days (64.9%) than the sham group (47.3%), though not statistically significant in the unadjusted analysis (OR 2.03 [95% CI 0.98-4.21], p = 0.057 However, statistical significance was achieved in the post-hoc analysis adjusted for age, baseline status (determined by pre-stroke mRS score), and initial stroke severity (measured by baseline RACE score by paramedics) (OR 2.94 [95% CI 1.21-7.16], p = 0.017). No serious adverse events were observed.
Interpretation: Despite the small sample size, our findings suggest that prehospital application of RIPerC is safe and may confer clinical benefit, as indicated in the post hoc adjusted analysis. However, larger, adequately powered trials are required to validate these results, and to determine potential differential effects across underrepresented patient subgroups.
Funding: Institute of Health Carlos III (ISCIII) of the Spanish Ministry of Health and Government of Catalonia-Agència de Gestió d'Ajuts Universitaris i de Recerca (AGAUR).
Keywords: Acute ischaemic stroke; Clinical trial; Neuroprotection; Remote ischaemic conditioning; Remote ischaemic preconditioning.
© 2025 The Authors.
Conflict of interest statement
We declare no competing interests.
Figures


References
-
- Purroy F., Montala N. Epidemiology of stroke in the last decade: a systematic review. Rev Neurol. 2021;73(9):321–336. - PubMed
-
- GBD 2021 Stroke Risk Factor Collaborators Global, regional, and national burden of stroke and its risk factors, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024;23(10):973–1003. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

