Monosensitization vs Polysensitization in Severe Asthma: Implications for Disease Severity
- PMID: 40337207
- PMCID: PMC12056521
- DOI: 10.2147/JAA.S502442
Monosensitization vs Polysensitization in Severe Asthma: Implications for Disease Severity
Abstract
Aim: The impact of sensitization on asthma outcomes in adults is still being discussed. This study aims to describe the sensitization profiles and allergic comorbidities of patients with severe asthma, and to analyze their association with asthma severity.
Patients and methods: This retrospective study included adult patients, evaluated at the Severe Asthma Clinic of Bichat University Hospital (Paris, France) during a 1-day hospital stay between May 2022 and January 2024. Sensitization, defined by a positive skin prick test and/or allergen-specific IgE levels greater than 0.10 kUA/L, was analysed alongside allergic comorbidities. The ASSESS score was used to grade asthma severity.
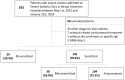
Results: Of the 201 patients included, 142 (70.6%) exhibited at least one sensitization to an aeroallergen, of whom 38 (26.8%) were monosensitized, and 104 (73.2%) were polysensitized. Compared to polysensitized patients, monosensitized patients were older at diagnosis (years: 30.6 ± 20.1 vs 21.7 ± 17.6, p = 0.01), had a higher ASSESS score (median (Q1; Q3); 13 (11; 15) vs 11 (9; 14), p = 0.02), a lower pre-bronchodilator forced expiratory volume in 1 second (%pred: 70.3 ± 23.2 vs 79.3 ± 21.8, p = 0.03), and experienced a greater burden of exacerbations (p = 0.03). There were significantly more polysensitized patients with at least three allergic comorbidities, but the number of allergic comorbidities did not correlate with asthma severity.
Conclusion: Monosensitized patients exhibited more severe disease and greater airway obstruction compared to polysensitized individuals. These findings suggest that allergies, especially in cases of late-onset asthma, may not be a significant determinant of asthma severity in adults.
Keywords: ASSESS; aeroallergens; allergy; sensitization; severe asthma.
© 2025 Gueçamburu et al.
Conflict of interest statement
CTDM has received grants or contracts from LFB Biomedicaments, payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from GSK and AstraZeneca, support for attending meetings and/or travel from ALK and AstraZeneca. LB has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Sanofi, GSK and support for attending meetings and/or travel from Sanofi. FL has received support for attending meetings and/or travel from ALK, Stallergènes, GSK, Sanofi, Novartis. CN has received in past 36 months honoraria for being a speaker or advisory board from AstraZeneca, Sanofi, ALK Abello, Stallergenes Greer, GSK, Viatris. CT has received lecture or advisory board fees and grants from AstraZeneca, Sanofi, GSK, Chiesi, Stallergenes Greer and Novartis. CD reports personal fees, non-financial support from Astra Zeneca, personal fees, non-financial support from Chiesi, personal fees, non-financial support from GSK, personal fees, non-financial support from Novartis, personal fees, non-financial support from Sanofi, outside the submitted work. Dr Catherine Neukirch reports personal fees from Viatris, personal fees from Stallergenes Greer, personal fees from ALK Abello, personal fees from AstraZeneca, personal fees from Sanofi, personal fees from GSK, personal fees from Zambon, outside the submitted work. The authors declare that they have no other conflicts of interest.
Figures
References
LinkOut - more resources
Full Text Sources