Skeletal muscle microvascular hemodynamic responses during hyperinsulinemic-euglycemic clamp in a Zucker Diabetic Sprague Dawley rat model of type 2 diabetes
- PMID: 40337247
- PMCID: PMC12055766
- DOI: 10.3389/fphys.2025.1568145
Skeletal muscle microvascular hemodynamic responses during hyperinsulinemic-euglycemic clamp in a Zucker Diabetic Sprague Dawley rat model of type 2 diabetes
Abstract
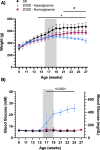
Objective: We sought to measure skeletal muscle microvascular hemodynamic responses in Sprague Dawley (SD) and Zucker Diabetic Sprague Dawley (ZDSD) rat model of type 2 diabetes (T2D) at rest and during a hyperinsulinemic-euglycemic clamp under resting conditions and during acute changes in local tissue oxygen concentration [(O2)].
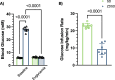
Methods: Male SD and ZDSD rats were fed a high-fat diet, transitioned to a high-fat high-sugar diet from 16-19 weeks old to induce T2D in the ZDSD strain, then returned to the high-fat diet until intravital video microscopy (IVVM). At 27 weeks of age animals were fasted overnight, and on the morning of the IVVM experiment animals were anaesthetized, instrumented, and mechanically ventilated. The extensor digitorum longus muscle was blunt dissected, isolated, and reflected over a glass coverslip or a gas exchange chamber (GEC) fitted in the stage of an inverted microscope. Microvascular hemodynamic responses were recorded during baseline and hyperinsulinemic-euglycemic clamp without perturbation (Protocol 1) and during sequential changes in GEC [O2] (7%-12%-2%-7%) (Protocol 2).
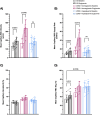
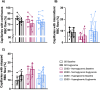
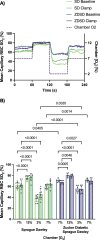
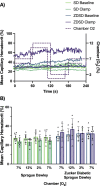
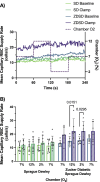
Results: In protocol 1, SD rats had a significant increase in red blood cell (RBC) velocity, RBC supply rate (SR), and RBC oxygen saturation (SO2) between baseline and euglycemia. However, ZDSD animals had no significant difference in hemodynamic responses and RBC SO2 between baseline and during hyperinsulinemic-euglycemic clamp. RBC SO2 was significantly higher in ZDSD than SD rats at baseline. In protocol 2, ZDSD rats had significantly higher RBC SO2 than their SD counterparts at 7% and 2% [O2]. RBC velocity, SR and capillary hematocrit showed no change from 7% in response to increased or decreased [O2] in either animal group. ZDSD rats had a significant increase between baseline and clamp in RBC SR at 12% as well as at 2% GEC [O2].
Conclusion: SD rats had a robust increase in capillary hemodynamics during hyperinsulinemic-euglycemic clamp whereas the capillary hemodynamics in ZDSD rats did not significantly change. Additionally, SD and ZDSD rats lacked expected hemodynamic responses in response to local [O2] changes during baseline and hyperinsulinemic-euglycemic clamp. This finding suggests that hyperglycemia in T2D and high-fat feeding alter microvascular hemodynamic responses to acute changes in muscle [O2].
Keywords: capillary hemodynamics; microvascular blood flow; oxygen mediated blood flow regulation; type 2 diabetes; zucker diabetic sprague dawley rat.
Copyright © 2025 Russell McEvoy, Wells, Kiley, Shogan and Fraser.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Bateman R. M., Jagger J. E., Sharpe M. D., Ellsworth M. L., Mehta S., Ellis C. G. (2001). Erythrocyte deformability is a nitric oxide-mediated factor in decreased capillary density during sepsis. Am. J. Physiol. Heart Circ. Physiol. 280 (6), H2848–H2856. 10.1152/ajpheart.2001.280.6.H2848 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

