From genetic code to global health: the impact of nucleic acid vaccines on disease prevention and treatment
- PMID: 40337306
- PMCID: PMC12053015
- DOI: 10.1039/d5md00032g
From genetic code to global health: the impact of nucleic acid vaccines on disease prevention and treatment
Abstract
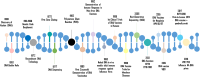
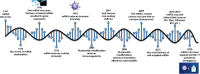
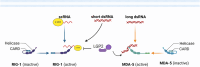
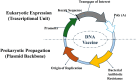
Vaccinology has revolutionized modern medicine, delivering groundbreaking solutions to prevent and control infectious diseases while pioneering innovative strategies to tackle non-infectious challenges, including cancer. Traditional vaccines faced inherent limitations, driving the evolution of next-generation vaccines such as subunit vaccines, peptide-based vaccines, and nucleic acid-based platforms. Among these, nucleic acid-based vaccines, including DNA and mRNA technologies, represent a major innovation. Pioneering studies in the 1990s demonstrated their ability to elicit immune responses by encoding specific antigens. Recent advancements in delivery systems and molecular engineering have overcome initial challenges, enabling their rapid development and clinical success. This review explores nucleic acid-based vaccines, including chemically modified variants, by examining their mechanisms, structural features, and therapeutic potential, while underscoring their pivotal role in modern immunization strategies and expanding applications across contemporary medicine.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There is no conflict of interest to declare.
Figures
























References
Publication types
LinkOut - more resources
Full Text Sources

