On the Hunt for Chiral Single-Atom Catalysts
- PMID: 40337368
- PMCID: PMC12053953
- DOI: 10.1021/acscatal.4c07405
On the Hunt for Chiral Single-Atom Catalysts
Abstract
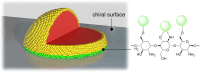
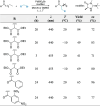
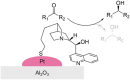
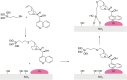
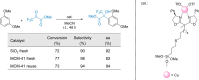
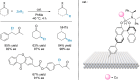
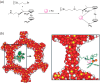
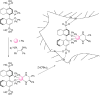
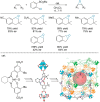
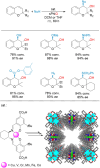
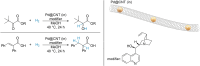
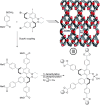
Enantioselective transformations are crucial in various fields, including chemistry, biology, and materials science. Today, the selective production of enantiopure compounds is achieved through asymmetric homogeneous catalysis. Single-atom catalysts (SACs) are emerging as a transformative approach in chemistry, enabling the heterogenization of organometallic complexes and effectively bridging the gap between homogeneous and heterogeneous catalysis. Despite their potential, the integration of SACs into enantioselective processes remains an underexplored area. This perspective offers a comprehensive analysis of possible strategies for the design of heterogeneous asymmetric catalysts, examining how chiral surfaces, chiral modifiers, grafted chiral complexes, and spatial confinement techniques can be effectively employed to enhance enantioselectivity. Each of these methods presents distinct advantages and challenges; for example, chiral surfaces and chiral modifiers offer potential for tailored reactivity but can suffer from limited stability and selectivity, while grafted chiral complexes provide robust platforms but may face issues related to scalability and synthesis complexity. Spatial confinement strategies show promise in enhancing catalyst efficiency but may be constrained by accessibility and reproducibility concerns. These strategies lay the groundwork for their adaptation to SACs, by providing innovative approaches to replicate the well-defined chiral environments of homogeneous catalysts while preserving the stability, reusability, and unique advantages of single-atom heterogeneous systems.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Catalysis without Precious Metals; Bullock R. M., Ed.; Wiley, 2010.
-
- Pasteur L. Mémoire Sur La Relation Qui Peut Exister Entre La Forme Crystalline et La Composition Chimique, et Sur La Cause de La Polarisation Rotatoire. C. R. Séances Acad. Sci. 1848, 26, 535–538.
-
- Sheldon R. A. The E Factor 25 Years on: The Rise of Green Chemistry and Sustainability. Green Chem. 2017, 19 (1), 18–43. 10.1039/C6GC02157C. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
