Lateral habenula astroglia modulate the potentiating antidepressant-like effects of bright light stimulation in intractable depression
- PMID: 40337515
- PMCID: PMC12055791
- DOI: 10.3389/fphar.2025.1592909
Lateral habenula astroglia modulate the potentiating antidepressant-like effects of bright light stimulation in intractable depression
Abstract
Background: Beside image vision, light plays a pivotal role in regulating diverse non-visual functions, including affective behaviors. Recently, bright light stimulation (BLS) was revealed to be beneficial for treating non-seasonal depression, although its mechanism of action is not fully understood.
Methods: We developed a novel mouse model of refractory depression, induced through social isolation and chronic despair during the active (dark) phase of the animal, and we have tested if antidepressant treatments, including BLS, could protect against anxio-depressive-like behavior.
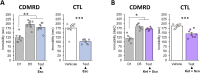
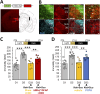
Results: We report that anxiety- and depressive-like behaviors are resistant to BLS as well as to both conventional and new antidepressants, including ketamine. Remarkably, we unveil that BLS potentiates the effect of antidepressants, and this beneficial effect is mediated via rod retinal photoreceptors. Furthermore, we demonstrate that both chemogenetic activation of lateral habenula (LHb) astroglia and serotonin (5-HT) depletion prevent the potentiating effect of BLS on chronic despair.
Conclusion: These results reveal, for the first time, that BLS enhances the efficacy of antidepressants through an unexpectedly circuit involving rods, LHb astroglia and 5-HT.
Keywords: animal model; bright light stimulation; chemogenetic; ketamine; refractory depression; rods.
Copyright © 2025 Delcourte, Bouloufa, Rovera, Brunet, Le, Williams, Panda, Azmani, Raineteau, Dkhissi-Benyahya and Haddjeri.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Dorsal raphe projection inhibits the excitatory inputs on lateral habenula and alleviates depressive behaviors in rats.Brain Struct Funct. 2018 Jun;223(5):2243-2258. doi: 10.1007/s00429-018-1623-3. Epub 2018 Feb 19. Brain Struct Funct. 2018. PMID: 29460052
-
A Visual Circuit Related to Habenula Underlies the Antidepressive Effects of Light Therapy.Neuron. 2019 Apr 3;102(1):128-142.e8. doi: 10.1016/j.neuron.2019.01.037. Epub 2019 Feb 19. Neuron. 2019. PMID: 30795900
-
Burst firing in Output-Defined Parallel Habenula Circuit Underlies the Antidepressant Effects of Bright Light Treatment.Adv Sci (Weinh). 2024 Aug;11(30):e2401059. doi: 10.1002/advs.202401059. Epub 2024 Jun 11. Adv Sci (Weinh). 2024. PMID: 38863324 Free PMC article.
-
[Habenula--a new target for treatment of intractable depression].Sheng Li Ke Xue Jin Zhan. 2011 Dec;42(6):407-12. Sheng Li Ke Xue Jin Zhan. 2011. PMID: 22363977 Review. Chinese.
-
Lateral Habenular Burst Firing as a Target of the Rapid Antidepressant Effects of Ketamine.Trends Neurosci. 2019 Mar;42(3):179-191. doi: 10.1016/j.tins.2018.12.002. Trends Neurosci. 2019. PMID: 30823984 Review.
References
-
- Bartlang M. S., Neumann I. D., Slattery D. A., Uschold-Schmidt N., Kraus D., Helfrich-Förster C., et al. (2012). Time matters: pathological effects of repeated psychosocial stress during the active, but not inactive, phase of male mice. J. Endocrinol. 215, 425–437. 10.1530/JOE-12-0267 - DOI - PubMed
LinkOut - more resources
Full Text Sources

