SHP2-mediated ROS activation induces chondrocyte paraptosis in osteoarthritis and is attenuated by low-intensity pulsed ultrasound
- PMID: 40337549
- PMCID: PMC12056802
- DOI: 10.1016/j.jot.2025.04.005
SHP2-mediated ROS activation induces chondrocyte paraptosis in osteoarthritis and is attenuated by low-intensity pulsed ultrasound
Abstract
Background: Paraptosis is a novel form of programmed cell death, generally caused by disrupted proteostasis or alterations of redox homeostasis. However, its impact and underlying mechanisms on the pathology of osteoarthritis (OA) are still unclear. This study aimed to investigate the role and regulatory mechanism of SHP2 in chondrocyte paraptosis and the effects influenced by low-intensity pulsed ultrasound (LIPUS).
Methods: SHP2, a MAPK upstream intermediary, has been identified as one of the critical targets of IL-1β-induced paraptosis in the GEO and GeneCard databases. The expression of SHP2 in chondrocytes was regulated by either siRNA knockdown or plasmid overexpression. Additionally, adeno-associated viruses were injected into the knee joints of rats to explore whether SHP2 plays a role in the development of OA. The impact of LIPUS on paraptosis and OA was examined in IL-1β-induced chondrocytes and a post-traumatic OA model, with SHP2 regulation assessed at both cellular and animal levels.
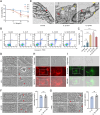
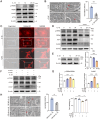
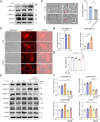
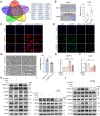
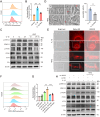
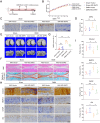
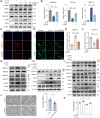
Results: An increase in cellular reactive oxygen species (ROS) caused by IL-1β halts the growth of chondrocytes and induces paraptosis in the chondrocytes. IL-1β-induced paraptosis, manifested as endoplasmic reticulum (ER)-derived vacuolization, was mediated by ROS-mediated ER stress and MAPK activation. SHP2 facilitates ROS production, thereby exacerbating the chondrocytes paraptosis. SHP2 knockdown and ROS inhibition effectively reduced this process and significantly mitigated inflammation and cartilage degeneration. Furthermore, we discovered that LIPUS delayed OA progression by inhibiting the activation of the MAPK pathway, ER stress, and ER-derived vacuoles in chondrocytes, all of which play critical roles in paraptosis, through the downregulation of SHP2 expression. Results on animals showed that LIPUS inhibited cartilage degeneration and alleviated OA progression.
Conclusion: SHP2 exacerbates IL-1β-induced oxidative stress and the subsequent paraptosis in chondrocytes, promoting OA progression. LIPUS mitigates paraptosis by modulating SHP2, which in turn slows OA progression.
The translational potential of this article: This study indicates that a novel SHP2-mediated cell death mechanism, paraptosis, plays a role in post-traumatic OA progression. LIPUS helps maintain cartilage-subchondral bone unit integrity by targeting SHP2 inhibition. SHP2 emerges as a potential therapeutic target, while LIPUS provides a promising non-invasive approach for treating trauma-related OA.
Keywords: Chondrocyte; LIPUS; Oxidative stress; Paraptosis; Post-traumatic OA; SHP2.
© 2025 The Authors.
Conflict of interest statement
The authors have declared that no competing interest exists.
Figures
Similar articles
-
Low-intensity pulsed ultrasound delays the progression of osteoarthritis by regulating the YAP-RIPK1-NF-κB axis and influencing autophagy.J Transl Med. 2024 Mar 16;22(1):286. doi: 10.1186/s12967-024-05086-x. J Transl Med. 2024. PMID: 38493143 Free PMC article.
-
Low-intensity pulsed ultrasound inhibits chondrocyte senescence by inhibiting PI3K/AKT/mTOR signaling.Braz J Med Biol Res. 2025 Mar 24;58:e14408. doi: 10.1590/1414-431X2025e14408. eCollection 2025. Braz J Med Biol Res. 2025. PMID: 40136230
-
Low-Intensity Pulsed Ultrasound Affects Chondrocyte Extracellular Matrix Production via an Integrin-Mediated p38 MAPK Signaling Pathway.Ultrasound Med Biol. 2015 Jun;41(6):1690-700. doi: 10.1016/j.ultrasmedbio.2015.01.014. Epub 2015 Feb 27. Ultrasound Med Biol. 2015. PMID: 25736607
-
Reactive oxygen species, aging and articular cartilage homeostasis.Free Radic Biol Med. 2019 Feb 20;132:73-82. doi: 10.1016/j.freeradbiomed.2018.08.038. Epub 2018 Aug 31. Free Radic Biol Med. 2019. PMID: 30176344 Free PMC article. Review.
-
Research Progress of Osteoarthritis Treatment by Low Intensity Pulsed Ultrasound.Smart Med. 2025 May 16;4(2):e70003. doi: 10.1002/smmd.70003. eCollection 2025 Jun. Smart Med. 2025. PMID: 40390768 Free PMC article. Review.
Cited by
-
"Multidisciplinary synergy driving innovation in orthopaedic translational medicine".J Orthop Translat. 2025 Jun 4;52:A1-A3. doi: 10.1016/j.jot.2025.06.001. eCollection 2025 May. J Orthop Translat. 2025. PMID: 40698066 Free PMC article. No abstract available.
References
-
- Peng Z., Sun H., Bunpetch V., Koh Y., Wen Y., Wu D., et al. The regulation of cartilage extracellular matrix homeostasis in joint cartilage degeneration and regeneration. Biomaterials. 2021;268 - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

