Evaluation of a Breath-Indicating Device for Enhanced Respiratory Monitoring and Apnea Detection in Postoperative Care: A Comparative Study
- PMID: 40337560
- PMCID: PMC12058232
- DOI: 10.7759/cureus.81846
Evaluation of a Breath-Indicating Device for Enhanced Respiratory Monitoring and Apnea Detection in Postoperative Care: A Comparative Study
Abstract
Objectives: Accurate respiratory monitoring is crucial in post-anesthetic care settings due to increased risks of respiratory complications. This study evaluates the impact of a new breath-indicating device, ApnoLight (PEMDx Pty Ltd, Brisbane, Queensland, Australia), on medical staff's accuracy in recording respiratory rates and detecting apneic events, along with the device's acceptance among nurses.
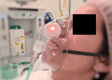
Methods: Twenty-five nurses from a hospital in Brisbane, Australia, participated. A simulated patient was fitted with the ApnoLight device on an oxygen mask. Nurses conducted six respiratory rate observations at varying distances (bedside, two meters, and five meters), both with and without the device. The patient's respiratory rates varied from eight to 25 breaths per minute. The accuracy of respiratory rate recordings and the time to identify apnea events were compared between simple observation and device-assisted observations.
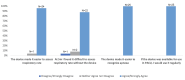
Results: The ApnoLight significantly reduced error rates in respiratory rate recordings: 27.58% at the bedside (P = 0.09, t = 1.31), 90.99% at two meters (P = 0.02, t = 1.98), and 96.37% at five meters (P = 0.0002, t = 4.02). The mean time to identify apnea decreased from 12.96 ± 9.12 seconds (simple observation) to 7.42 ± 2.19 seconds (with ApnoLight device). All apnea events were identified with the device, whereas four were undetected without it. Feedback showed that 96% (N = 24) of nurses found the device improved respiratory rate accuracy, and 100% (N = 25) found it made apnea identification easier.
Conclusions: The ApnoLight device has the potential to enhance respiratory rate monitoring accuracy and apnea detection in postoperative settings. Its implementation could improve patient safety and streamline clinical workflows.
Keywords: apnea detection; breath-indicating device; continuous monitoring; perioperative care; respiratory monitoring.
Copyright © 2025, Rowe et al.
Conflict of interest statement
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study. The Uniting Care Human Research Ethics Committee issued approval 2020.18.328. Human ethics review committee approval was obtained from the Uniting Care Human Research Ethics Committee for subject recruitment and data analysis. The authors do not know of any ethical issues to declare. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Intellectual property info: Drs. Mariam Rowe and Phillip Melksham and Mr. Dylan Rowe jointly own intellectual property on the ApnoLight technology (US20230201516A1) and jointly own shares in PEMDx Pty Ltd. Mr. Dylan Stubbs and Mr. Chase Pontifex have no conflicts of interest to declare. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
Similar articles
-
[Standard technical specifications for methacholine chloride (Methacholine) bronchial challenge test (2023)].Zhonghua Jie He He Hu Xi Za Zhi. 2024 Feb 12;47(2):101-119. doi: 10.3760/cma.j.cn112147-20231019-00247. Zhonghua Jie He He Hu Xi Za Zhi. 2024. PMID: 38309959 Chinese.
-
Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine.J Clin Sleep Med. 2012 Oct 15;8(5):597-619. doi: 10.5664/jcsm.2172. J Clin Sleep Med. 2012. PMID: 23066376 Free PMC article.
-
A New Approach for Detecting Sleep Apnea Using a Contactless Bed Sensor: Comparison Study.J Med Internet Res. 2020 Sep 18;22(9):e18297. doi: 10.2196/18297. J Med Internet Res. 2020. PMID: 32945773 Free PMC article.
-
Prospective Observational Investigation of Capnography and Pulse Oximetry Monitoring After Cesarean Delivery With Intrathecal Morphine.Anesth Analg. 2019 Mar;128(3):513-522. doi: 10.1213/ANE.0000000000003503. Anesth Analg. 2019. PMID: 29958217
-
Development of three methods for extracting respiration from the surface ECG: a review.J Electrocardiol. 2014 Nov-Dec;47(6):819-25. doi: 10.1016/j.jelectrocard.2014.07.020. Epub 2014 Aug 4. J Electrocardiol. 2014. PMID: 25194875 Review.
References
-
- Role of continuous pulse oximetry and capnography monitoring in the prevention of postoperative respiratory failure, postoperative opioid-induced respiratory depression and adverse outcomes on hospital wards: a systematic review and meta-analysis. Khanna AK, Banga A, Rigdon J, et al. J Clin Anesth. 2024;94:111374. - PubMed
-
- Systems of care delivery and optimization in the postoperative care wards. Snarskis C, Banerjee A, Franklin A, Weavind L. Anesthesiol Clin. 2023;41:875–886. - PubMed
-
- Postoperative day one: a high risk period for respiratory events. Taylor S, Kirton OC, Staff I, Kozol RA. Am J Surg. 2005;190:752–756. - PubMed
-
- Respiratory complications of anaesthesia. Mills GH. Anaesthesia. 2018;73 Suppl 1:25–33. - PubMed
LinkOut - more resources
Full Text Sources