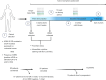
A new dawn in cancer immunotherapy: the promise of mutant KRAS-specific vaccines
- PMID: 40337764
- PMCID: PMC12056101
- DOI: 10.21037/tgh-24-121
A new dawn in cancer immunotherapy: the promise of mutant KRAS-specific vaccines
Keywords: Cancer vaccine; KRAS; colorectal cancer (CRC); pancreatic ductal adenocarcinoma (PDA).
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-121/coif). V.G.P. reports lab research funding from NGM, OncoResponse and Merck and payment from Sensei, Umoja and TriSalus. R.A.S. reports research funding (paid to the institution) by Verastem, Exelixis, Replimune as well as the V Foundation, and advisory board member for Ipsen, Guardant Health, and Agenus. E.G.C. reports advisory board role for Ipsen, Merus, Pfizer, BMS, Takeda, Regeneron, BPGBio, IGM Biosciences, G1 Therapeutics, Merck, Novartis, Astellas, and Foundation Medicine; and research funding, paid to the institution, by Lonza, AffiniT, Erasca, BMS, Novartis, Gilead, Merck, Roche, AADi, Boehringer-Ingelheim, Fibrogen, Cornerstone, Biosplice. The other author has no conflicts of interest to declare.
Figures
Comment on
-
Lymph-node-targeted, mKRAS-specific amphiphile vaccine in pancreatic and colorectal cancer: the phase 1 AMPLIFY-201 trial.Nat Med. 2024 Feb;30(2):531-542. doi: 10.1038/s41591-023-02760-3. Epub 2024 Jan 9. Nat Med. 2024. PMID: 38195752 Free PMC article. Clinical Trial.
References
-
- Gong Z, Esmail A, Abdelrahim M. Circulating tumor DNA (ctDNA) and disease recurrence in early stage pancreatic cancer. J Clin Oncol 2023;41:e16313. 10.1200/JCO.2023.41.16_suppl.e16313 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous