Colonic inflammation triggers β cell proliferation during obesity development via a liver-to-pancreas interorgan mechanism
- PMID: 40337860
- PMCID: PMC12128978
- DOI: 10.1172/jci.insight.183864
Colonic inflammation triggers β cell proliferation during obesity development via a liver-to-pancreas interorgan mechanism
Abstract
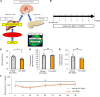
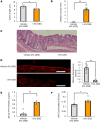
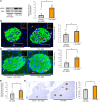
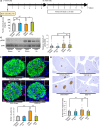
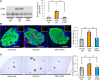
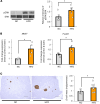
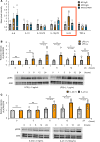
Under insulin-resistant conditions, such as obesity, pancreatic β cells adaptively proliferate and secrete more insulin to prevent blood glucose elevation. We previously reported hepatic ERK activation during obesity development to stimulate a neuronal relay system, consisting of afferent splanchnic nerves from the liver and efferent vagal nerves to the pancreas, thereby triggering adaptive β cell proliferation. However, the mechanism linking obesity with the interorgan system originating in hepatic ERK activation remains unclear. Herein, we clarified that colonic inflammation promotes β cell proliferation through this interorgan system from the liver to the pancreas. First, dextran sodium sulfate (DSS) treatment induced colonic inflammation and hepatic ERK activation as well as β cell proliferation, all of which were suppressed by blockades of the neuronal relay system by several approaches. In addition, treatment with anti-lymphocyte Peyer's patch adhesion molecule-1 (anti-LPAM1) antibody suppressed β cell proliferation induced by DSS treatment. Importantly, high-fat diet (HFD) feeding also elicited colonic inflammation, and its inhibition by anti-LPAM1 antibody administration suppressed hepatic ERK activation and β cell proliferation induced by HFD. Thus, colonic inflammation triggers adaptive β cell proliferation via the interorgan mechanism originating in hepatic ERK activation. The present study revealed a potentially novel role of the gastrointestinal tract in the maintenance of β cell regulation.
Keywords: Beta cells; Endocrinology; Metabolism; Obesity.
Conflict of interest statement
Figures







References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

