CIAPIN1 promotes proliferation and migration of PDGF-BB-activated airway smooth muscle cells via the PI3K/AKT and JAK2/STAT3 signaling pathways
- PMID: 40338178
- PMCID: PMC12058325
- DOI: 10.14814/phy2.70360
CIAPIN1 promotes proliferation and migration of PDGF-BB-activated airway smooth muscle cells via the PI3K/AKT and JAK2/STAT3 signaling pathways
Abstract
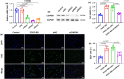
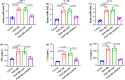
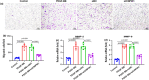
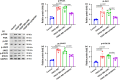
Cytokine-induced apoptosis inhibitor 1 (CIAPIN1) is an essential anti-apoptotic protein; however, its role and associated molecular pathways in asthma remain largely unexplored. This study aimed to investigate the potential effects of CIAPIN1 on the proliferation and migration of platelet-derived growth factor BB (PDGF-BB)-induced ASMCs and the underlying mechanisms involved. Considering these aspects, ASMCs are activated with PDGF-BB as a cellular model for asthma. CIAPIN1 is then downregulated using small interfering ribonucleic acid (siRNA). Western blot analysis was performed to assess protein expression. Elevated levels of CIAPIN1 were observed, demonstrating a positive correlation with cytokine levels. CIAPIN1 expression is significantly increased in PDGF-BB-induced human ASMCs. In addition, CIAPIN1 knockdown inhibited proliferation, inflammatory cytokine production, and migration ability, while elevating apoptosis in PDGF-BB-induced human ASMCs. Moreover, CIAPIN1 knockdown inhibited phosphorylated phosphoinositide 3-kinase (p-PI3K), phosphorylated protein kinase B (p-Akt), phosphorylated Janus kinase 2 (p-JAK2), and phosphorylated signal transducer and activator of transcription 3 (p-STAT3) protein expression. In conclusion, the results indicate that CIAPIN1 regulates the proliferation and migration of human ASMC in response to PDGF-BB by inhibiting the PI3K/AKT and JAK2/STAT3 pathways.
Keywords: CIAPIN1; JAK2/STAT3; PI3K/AKT; asthma; migration; proliferation.
© 2025 The Author(s). Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


References
-
- Abe, Y. , Ono, K. , Kawamura, T. , Wada, H. , Kita, T. , Shimatsu, A. , & Hasegawa, K. (2007). Leptin induces elongation of cardiac myocytes and causes eccentric left ventricular dilatation with compensation. American Journal of Physiology. Heart and Circulatory Physiology, 292(5), 2387–2396. - PubMed
-
- Banno, A. , Reddy, A. T. , Lakshmi, S. P. , & Reddy, R. C. (2020). Bidirectional interaction of airway epithelial remodeling and inflammation in asthma. Clinical Science (London, England), 134(9), 1063–1079. - PubMed
-
- Förster, K. , Paul, I. , Solenkova, N. , Staudt, A. , Cohen, M. V. , Downey, J. M. , Felix, S. B. , & Krieg, T. (2006). NECA at reperfusion limits infarction and inhibits formation of the mitochondrial permeability transition pore by activating p70S6 kinase. Basic Research in Cardiology, 101(4), 319–326. - PubMed
-
- Gao, P. , Ding, Y. , Yin, B. , & Gu, H. (2021). Long noncoding RNA LINC‐PINT retards the abnormal growth of airway smooth muscle cells via regulating the microRNA‐26a‐5p/PTEN axis in asthma. International Immunopharmacology, 99, 107997. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

