Peptide-Based Regulation of TNF-α-Mediated Cytotoxicity
- PMID: 40338229
- PMCID: PMC12024540
- DOI: 10.3390/biom15040559
Peptide-Based Regulation of TNF-α-Mediated Cytotoxicity
Abstract
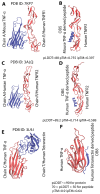
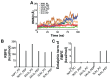
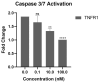
Tumor necrosis factor alpha (TNF-α) is a pro-inflammatory cytokine associated with TNF receptor 1 (TNFR1) and TNF receptor 2 (TNFR2), which play important roles in several inflammatory diseases. There is a growing interest in developing alternative molecules that can be used as TNF blockers. In this study, we focused on TNF-α-, TNFR1-, and TNFR2-mimicking peptides to inhibit TNF-α receptor binding in various ways. Six peptides (OB1, OB2, OB5, OB6, OB7, and OB8) were developed to bind TNFR1, TNFR2, and TNF-α. OB1 and OB2 bound to TNF-α with lower Kd values of 300 and 46.7 nM, respectively, compared to previously published sequences. These synthetic peptides directly and indirectly inhibited TNF-α in vitro without cytotoxicity to L929 cells, and OB1 significantly inhibited apoptosis in the presence of hTNF-α. Peptides developed in this study may prove to be useful for therapeutic inhibition of TNF-α.
Keywords: TNF-α; TNF-α blocker; TNF-α inhibition; TNF-α receptors; TNF-α-binding peptide; TNFR1-binding peptide; TNFR2-binding peptide.
Conflict of interest statement
Ahmet Emin Atik is employed by the company Turgut Ilaclari A.S. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

