Dupilumab Efficacy in Children with Atopic Dermatitis with Different Phenotypes and Endotypes: A Case Series
- PMID: 40338484
- PMCID: PMC12182520
- DOI: 10.1007/s12325-025-03150-6
Dupilumab Efficacy in Children with Atopic Dermatitis with Different Phenotypes and Endotypes: A Case Series
Abstract
Introduction: Atopic dermatitis (AD) is a prevalent disease in infants and young children worldwide. Dupilumab has been shown to rapidly and significantly improve AD signs, symptoms, and quality of life in pediatric patients with moderate-to-severe AD.
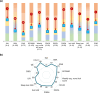
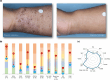
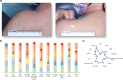
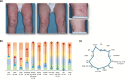
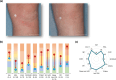
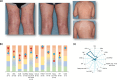
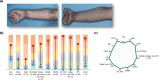
Methods: In LIBERTY AD PRESCHOOL, a randomized, double-blind, placebo-controlled, phase 3 clinical trial, patients aged 6 months to 5 years with moderate-to-severe AD received subcutaneous dupilumab or matched placebo every 4 weeks for 16 weeks. All patients received concomitant low-potency topical corticosteroids. Here, we present 12 photographic cases of patients with different phenotypes and endotypes in the dupilumab group, representative of the study population, before and after treatment. Each case is presented with clinical outcome measures of AD severity and quality of life, as well as relevant biomarkers, with percent improvement from baseline.
Results: Treatment with dupilumab led to visible improvements in signs of lichenification, erythema, excoriations, skin dryness, and oozing/crusting. Clinically meaningful improvements in the measured outcomes were observed in most of the patients, including AD extent and severity, clinical lesions, itch, sleep loss, frequency of symptoms, and quality of life. These improvements were associated with substantial reductions in AD-related biomarkers.
Conclusion: Treatment with dupilumab improved signs, symptoms, and quality of life, and reduced AD-related serum biomarkers in young children with moderate-to-severe AD with different phenotypes and endotypes.
Trial registration: The trial was registered with ClinicalTrials.gov with ID number NCT03346434 on November 17, 2017. Video abstract available for this article. Video abstract (MP4 1,02,609 KB).
Keywords: Atopic dermatitis; Dupilumab; Endotypes; Pediatric dermatology; Phenotypes.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Ana B. Rossi and Adriana M. Mello are employees of and may hold stock and/or stock options in Sanofi. Joseph Zahn is an employee and shareholder of Regeneron Pharmaceuticals Inc. Ethical Approval: The study was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice guidelines, and applicable regulatory requirements. An independent data and safety monitoring committee conducted blinded monitoring of patient safety data. The local Institutional Review Board or Ethics Committee at each study center oversaw trial conduct and documentation. The study was approved by the Copernicus Group ethics committee. All parents/guardians provided written informed consent before participating in the trial, including for the publication of clinical photos taken in selected centers. Pediatric patients provided assent according to the Ethics Committee (Institutional Review Board/Independent Ethics Committee)-approved standard practice for pediatric patients at each participating center.
Figures






References
-
- Silverberg JI, Barbarot S, Gadkari A, Simpson EL, Weidinger S, Mina-Osorio P, et al. Atopic dermatitis in the pediatric population: a cross-sectional, international epidemiologic study. Ann Allergy Asthma Immunol. 2021;126:417-428.e2. 10.1016/j.anai.2020.12.020. - PubMed
-
- Weidinger S, Simpson EL, Silverberg JI, Barbarot S, Eckert L, et al. Burden of atopic dermatitis in paediatric patients: an international cross-sectional study. Br J Dermatol. 2023. 10.1093/bjd/ljad449. - PubMed
-
- Yang EJ, Beck KM, Sekhon S, Bhutani T, Koo J. The impact of pediatric atopic dermatitis on families: a review. Pediatr Dermatol. 2019;36:66–71. 10.1111/pde.13727. - PubMed
-
- Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396:345–60. 10.1016/S0140-6736(20)31286-1. - PubMed
-
- Barbarot S, Silverberg JI, Gadkari A, Simpson EL, Weidinger S, Mina-Osorio P, et al. The family impact of atopic dermatitis in the pediatric population: results from an international cross-sectional study. J Pediatr. 2022;246:220-226.e5. 10.1016/j.jpeds.2022.04.027. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

