Multicenter, Phase 4 Clinical Study from India to Evaluate the Safety and Efficacy of Insulin Glargine 300 U/ml in Insulin-Naïve People with Type 2 Diabetes Uncontrolled on Oral Anti-hyperglycemic Drugs: SAFEGUARD Study
- PMID: 40338497
- PMCID: PMC12182549
- DOI: 10.1007/s13300-025-01736-5
Multicenter, Phase 4 Clinical Study from India to Evaluate the Safety and Efficacy of Insulin Glargine 300 U/ml in Insulin-Naïve People with Type 2 Diabetes Uncontrolled on Oral Anti-hyperglycemic Drugs: SAFEGUARD Study
Abstract
Introduction: The clinical benefits of insulin glargine 300 U/ml (Gla-300) have been confirmed in randomized clinical trials (EDITION and BRIGHT) and real-world studies (ATOS, Toujeo-1, and ORION) in different regions of the world. However, safety data for the Indian population are lacking. The current post-marketing surveillance study evaluated the safety and efficacy of Gla-300 in people with type 2 diabetes (T2D) from India.
Methods: SAFEGUARD was a multicenter, phase 4, single-arm, open-label, 24-week study conducted at 15 centers across India between August 10, 2021 and December 26, 2022. The study included insulin-naïve participants (aged ≥ 18 years) with T2D uncontrolled (HbA1c ≥ 7.5% and ≤ 10%) on oral anti-hyperglycemic drugs. The primary endpoint was the percentage of participants with treatment-emergent adverse events (TEAEs), including serious adverse events (SAEs) and hypoglycemic episodes.
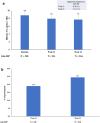
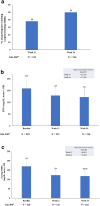
Results: Of the 220 participants included, 218 (36.8% female) were eligible for efficacy analysis. The mean ± standard deviation age was 54.0 ± 9.6 years, the baseline HbA1c was 8.8 ± 0.9%, and the duration of T2D was 9.3 ± 7.0 years. Among the 220 participants who took at least one dose of Gla-300, 24.5% (n = 54) reported 64 TEAEs, which included one (0.5%) SAE. The most reported event was infections (10.9%). In total, 29.5% of participants (n = 65) reported a level 1 hypoglycemic event, and 27.7% of participants (n = 18) reported the main symptom of sweating. Glycemic control improved with reductions in mean HbA1c levels (- 1.14 ± 1.2%), fasting plasma glucose (- 37.0 ± 59.3 mg/dl), and fasting self-monitored blood glucose (- 52.0 ± 44.1 mg/dl) from baseline to week 24.
Conclusions: Gla-300 was well tolerated with improved glycemic control and a low hypoglycemia risk in insulin-naïve people with T2D living in India.
Trial registration: Clinical Trials Registry-India (CTRI number): CTRI/2021/07/035244. WHO identifier number: U1111-1255-5143. NCT number: NCT04980027.
Keywords: Diabetes mellitus; Hypoglycemia; India; Insulin glargine; SAFEGUARD.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Viswanathan Mohan has acted as a consultant and speaker and received research or educational grants from Sanofi, Servier, Novo Nordisk, Abbott, Boehringer Ingelheim, Eli Lilly, Lifescan, Roche, MSD, Novartis, Aventis, Bayer, USV, Dr. Reddy’s Laboratories, Sun Pharma, INTAS, Lupin, Glenmark, Zydus, IPCA, Torrent, Cipla, Biocon, Primus, Franco Indian, Wockhardt, Emcure, Mankind, Medtronics, Fourrts, Apex, GSK, and Alembic. Bipin Sethi has been an advisory board member or received speaker fees from Abbot, Bayer, Boehringer Ingelheim, MSD, USV, and Novo Nordisk. Rakesh Sahay is an advisory board member for Boehringer Ingelheim, Dr. Reddy’s Laboratories, Eli Lilly, and Sanofi and speaker for Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Sanofi. Balamurugan Ramanathan, Sunil Jain, Sreenivasa Murthy, and Kiran Pal Singh have nothing to disclose. Shalini Menon was an employee of Sanofi at the time of study conduct and preparation of this manuscript. Shalini Menon’s current affiliation is – Medical Affairs, GlaxoSmithKline, Mumbai, India. Arvind Gadekar, Vaibhav Salvi, and Kedar Gandhi are employees of Sanofi and may hold stock options. Ethical Approval: This study was performed in compliance with the International Council for Haromonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Good Clinical Practice (GCP) and the principles laid down in the 1964 Declaration of Helsinki by the 18 th World Medical Assembly and its later amendments. The study is prospectively registered at the Clinical Trials Registry—India (CTRI number: CTRI/2021/07/035244) and at the International Clinical Trials Registry Platform – the WHO Registry Network (WHO identifier number: U1111 - 1255–5143). The study protocol was reviewed and approved by the Institutional Review Board/Institutional Ethics Committee at each study site in accordance with the country’s local regulations. All participants provided written informed consent.
Figures

References
-
- Symposium on “Recent Trends and Siddha Management management of diabetes”. National Institute of Siddha, Tambaram Sanatorium, Chennai: PIB Chennai; September 09, 2023. https://pib.gov.in/PressReleasePage.aspx?PRID=1955800#:~:text=According%....
-
- Anjana RM, Unnikrishnan R, Deepa M, et al. Metabolic non-communicable disease health report of India: the ICMR-INDIAB national cross-sectional study (ICMR-INDIAB-17). Lancet Diabetes Endocrinol. 2023;11(7):474–89. - PubMed
-
- American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2021. Diabetes Care. 2020;44(Supplement_1):S111–24. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

