Treatment Patterns and Survival Among Veterans With De Novo Metastatic Hormone-Sensitive Prostate Cancer
- PMID: 40338545
- PMCID: PMC12062907
- DOI: 10.1001/jamanetworkopen.2025.9433
Treatment Patterns and Survival Among Veterans With De Novo Metastatic Hormone-Sensitive Prostate Cancer
Abstract
Importance: Combination therapy for metastatic hormone-sensitive prostate cancer (mHSPC) has been widely adopted, yet clinical use and outcomes are unknown. Furthermore, optimal therapy is uncertain due to lack of direct comparison of androgen receptor pathway inhibitors (ARPIs) and docetaxel in high-volume disease.
Objective: To evaluate the use of combination therapy and its association with overall survival among patients with mHSPC and to compare ARPIs vs docetaxel doublet therapy by volume of disease.
Design, setting, and participants: This retrospective cross-sectional study was conducted in the US Veterans Health Administration among 6216 US veterans with de novo mHSPC from January 1, 2013, to December 31, 2022, treated with androgen deprivation therapy (ADT) within 3 months of diagnosis. Treatments for mHSPC were collected within 4 months of ADT. Volume of disease was assessed from radiology report review. Data were analyzed from July 2023 to October 2024.
Main outcomes and measures: Overall survival (OS) and clinical progression-free survival (PFS), indicated by time to castration resistance or death.
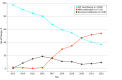
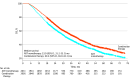
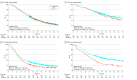
Results: Among 6216 male veterans with mHSPC (mean [SD] age, 73.9 [9.7] years), use of combination therapy increased from 344 of 637 veterans (54.0%) in 2020 to 465 of 737 veterans (63.1%) in 2022. Among 4106 veterans treated from 2017 to 2022, combination therapy was associated with longer OS (40.3 [95% CI, 38.0-42.1] months vs 33.0 [95% CI, 31.2-35.1] months; hazard ratio [HR], 0.80 [95% CI, 0.74-0.87]) and was used more frequently among younger veterans with fewer comorbidities. Among 1174 veterans with high-volume mHSPC, there was no difference in OS between ARPIs and docetaxel (32.3 [95% CI, 29.5-35.3] months vs 34.7 [95% CI, 31.7-37.1] months; HR, 1.06 [95% CI, 0.91-1.23]); however, ARPIs were associated with longer PFS (18.7 [95% CI, 17.1-20.9] months vs 16.0 [95% CI, 14.0-17.7] months; HR, 0.80 [95% CI, 0.70-0.91]; P = .001). In a multivariable model of high-volume mHSPC, there was no difference in OS between ARPIs and docetaxel (adjusted HR, 0.89 [95% CI, 0.76-1.05]). Among 366 veterans with low-volume mHSPC, there was no difference in OS between ARPIs and docetaxel (68.4 [95% CI, 52.6 months to not reached] months vs 55.3 [95% CI, 41.7-78.9] months; HR, 0.81 [95% CI, 0.58-1.13]), but ARPIs were associated with longer PFS (39.7 [95% CI, 34.3-52.9] months vs 24.0 [95% CI, 20.3-32.9] months; HR, 0.57 [95% CI, 0.43-0.76]).
Conclusions and relevance: In this cross-sectional study of veterans with de novo mHSPC, use of combination therapies increased over time and were associated with longer survival compared with ADT monotherapy. In both high- and low-volume mHSPC, no differences in OS were observed between ARPI and docetaxel combinations; however, ARPIs had longer PFS. Future research into the role of docetaxel is needed to elucidate the benefit of chemotherapy in mHSPC.
Conflict of interest statement
Figures

Comment in
References
-
- Sweeney C, Chen YH, Carducci MA, et al. Impact on overall survival (OS) with chemohormonal therapy versus hormonal therapy for hormone-sensitive newly metastatic prostate cancer (mPrCa): an ECOG-led phase III randomized trial. J Clin Oncol. 2014;32(18)(suppl):LBA2. doi: 10.1200/jco.2014.32.18_suppl.lba2 - DOI
-
- James ND, Sydes MR, Clarke NW, et al. ; STAMPEDE investigators . Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163-1177. doi: 10.1016/S0140-6736(15)01037-5 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

