Enterovirus D68-Associated Respiratory Illness in Children
- PMID: 40338550
- PMCID: PMC12062906
- DOI: 10.1001/jamanetworkopen.2025.9131
Enterovirus D68-Associated Respiratory Illness in Children
Abstract
Importance: Enterovirus D68 (EV-D68) typically causes mild to severe acute respiratory illness (ARI). Testing and surveillance for EV-D68 in the US are limited, and important epidemiologic gaps remain.
Objective: To characterize the epidemiology and clinical severity of EV-D68 among US children seeking care for ARI from 2017 to 2022, using a multisite, active, systematic surveillance network.
Design, setting, and participants: This cross-sectional study collected data from the New Vaccine Surveillance Network, an active, prospective, population-based surveillance system of emergency departments (EDs) and hospitals at 7 US academic medical centers. Children with ARI and EV-D68-positive results were enrolled during platform-wide EV-D68 testing periods (July to October 2017, July to November 2018, July to November 2020, and July 2021 to December 2022). Included children were aged younger than 18 years, reported 1 or more qualifying ARI symptoms, with a symptom duration less than 14 days at enrollment. Data were analyzed from in October 2024.
Exposures: Laboratory-confirmed EV-D68 infection, including overall infections or those without viral codetection.
Main outcomes and measures: Trends and characteristics of EV-D68, including demographics, underlying conditions, and clinical severity by health care setting, were explored. Among hospitalized children with EV-D68-positive results without viral codetection, multivariable logistic regression was used to examine factors associated with receipt of (1) supplemental oxygen or (2) intensive care.
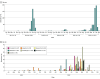
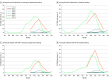
Results: From 2017 to 2022, 976 children with EV-D68-positive results were identified (median [IQR] age, 47 [18-63] months; 391 [40.1%] female); most were enrolled in 2018 (382 children) and 2022 (533 children). Among these, 856 had no viral codetection, of which 320 were discharged home from the ED (median [IQR] age, 33 [16-59] months; 180 male [56.3%]; 237 [74.1%] with no reported underlying conditions) and 536 were hospitalized (median [IQR] age, 40 [19-69] months; 330 male [61.6%]; 268 [50.0%] with no reported underlying conditions). Among those hospitalized, 199 (37.1%) reported a history of asthma or reactive airway disease (RAD) and 77 (14.4%) reported a condition other than asthma or RAD. Having an underlying condition other than asthma or RAD was associated with increased odds of receiving supplemental oxygen (adjusted odds ratio, 2.72; 95% CI, 1.43-5.18) or intensive care admission (adjusted odds ratio, 3.09; 95% CI, 1.72-5.56); neither age group nor history of asthma or RAD were associated with oxygen receipt or intensive care admission.
Conclusions and relevance: In this cross-sectional study of children with medically attended EV-D68 infections, EV-D68 was associated with severe disease in otherwise healthy children of all ages, and children with nonasthma or RAD comorbidities were at higher risk for severe outcomes when hospitalized.
Conflict of interest statement
Figures
References
-
- Midgley CM, Watson JT, Nix WA, et al. ; EV-D68 Working Group . Severe respiratory illness associated with a nationwide outbreak of enterovirus D68 in the USA (2014): a descriptive epidemiological investigation. Lancet Respir Med. 2015;3(11):879-887. doi: 10.1016/S2213-2600(15)00335-5 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

