Interim Evaluation of Respiratory Syncytial Virus Hospitalization Rates Among Infants and Young Children After Introduction of Respiratory Syncytial Virus Prevention Products - United States, October 2024-February 2025
- PMID: 40338822
- PMCID: PMC12061057
- DOI: 10.15585/mmwr.mm7416a1
Interim Evaluation of Respiratory Syncytial Virus Hospitalization Rates Among Infants and Young Children After Introduction of Respiratory Syncytial Virus Prevention Products - United States, October 2024-February 2025
Abstract
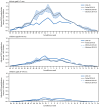
Maternal respiratory syncytial virus (RSV) vaccine and nirsevimab, a long-acting monoclonal antibody for infants aged 0-7 months and children aged 8-19 months who are at increased risk for severe RSV disease, became widely available for prevention of severe RSV disease among infants and young children during the 2024-25 RSV season. To evaluate the association between availability of these products and infant and child RSV-associated hospitalization rates, the rates among children aged <5 years were compared for the 2024-25 and 2018-20 RSV seasons using data from the RSV-Associated Hospitalization Surveillance Network (RSV-NET) and New Vaccine Surveillance Network (NVSN). Among infants aged 0-7 months (eligible for protection with maternal vaccination or nirsevimab), 2024-25 RSV-associated hospitalization rates were lower compared with 2018-20 pooled rates (estimated relative rate reductions of 43% [RSV-NET: 95% CI = 40%-46%] and 28% [NVSN: 95% CI = 18%-36%]). The largest estimated rate reduction was observed among infants aged 0-2 months (RSV-NET: 52%, 95% CI = 49%-56%; NVSN: 45%, 95% CI = 32%-57%) and during peak hospitalization periods (December-February). These findings support Advisory Committee on Immunization Practices' recommendations for maternal vaccination or nirsevimab to protect against severe RSV disease in infants and highlight the importance of implementing the recommendations to protect infants as early in the RSV season as possible, before peak transmission, and for infants born during the RSV season, within the first week of life, ideally during the birth hospitalization.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Ruth Lynfield reports receipt of a fee from the American Academy of Pediatrics Red Book (Report of the Committee on Infectious Diseases), which was donated to the Minnesota Department of Health. Brenda L. Tesini reports membership on the Merck Manuals Editorial Board. William Schaffner reports receipt of personal fees from Abbott Diagnostics for presentation of a lecture. Daniel C. Payne reports receipt of payment from Merck for Scientific Input Engagement participation. Mary A. Staat reports institutional support from the National Institutes of Health, Merck, Pfizer, and Cepheid; royalties from Up-to-Date; and consulting fees from Merck for norovirus epidemiology consult. Leila C. Sahni reports receipt of travel support from the Bill and Melinda Gates Foundation. Jennifer E. Schuster reports institutional support from the National Institutes of Health, the Food and Drug Administration, and the state of Missouri; receipt of a fee from the Association of Professionals in Infection Control and Epidemiology for consulting on educational curriculum; receipt of honoraria from the Missouri Academy of Pediatrics; and service on the advisory board of the Association of American Medical Colleges for a grant awarded for vaccine confidence. Rangaraj Selvarangan reports institutional support from BioMérieux, Cepheid, Hologic, Qiagen, Meridian, and Abbot; consulting fees from BioMérieux, Baebies, GSK, and Haleon for service on advisory boards; payment and support for meeting attendance from Abbot and BioMérieux; and payment from the American Society for Microbiology for participation on a conference organizing committee. Natasha B. Halasa reports receipt of grant support from Sanofi and Quidel, and honoraria from Genentech. Marian G. Michaels reports support for meeting attendance from the American Society of Transplantation for a talk on respiratory viruses, including respiratory syncytial virus (RSV). John V. Williams reports institutional support from the National Institutes of Health; payment for providing lectures from St. Jude Research Hospital and the American Pharmacists Association, and participation on a National Institutes of Health data safety monitoring board for the National Institute of Allergy and Infectious Diseases IMPAACT Study. Geoffrey A. Weinberg reports consulting fees from the New York State Department of Health and Inhalon Biopharma; honoraria from Merck & Co.; and participation on a Data Safety Monitoring Board at Emory University. Janet A. Englund reports institutional support from AstraZeneca; GSK, Merck, Pfizer, and Moderna; consulting fees from Abbvie, AstraZeneca, GSK, Merck, Meissa Vaccines, Moderna, Pfizer, and Sanofi Pasteur; and payment from Pfizer for delivering a presentation at an RSV meeting. Eileen J. Klein reports receipt of honoraria from Children’s Hospital of New Orleans for presenting grand rounds on research networks. No other potential conflicts of interest were disclosed.
Figures

References
-
- Jones JM, Fleming-Dutra KE, Prill MM, et al. Use of nirsevimab for the prevention of respiratory syncytial virus disease among infants and young children: recommendations of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb Mortal Wkly Rep 2023;72:920–5. 10.15585/mmwr.mm7234a4 - DOI - PMC - PubMed
-
- Fleming-Dutra KE, Jones JM, Roper LE, et al. Use of the Pfizer respiratory syncytial virus vaccine during pregnancy for the prevention of respiratory syncytial virus–associated lower respiratory tract disease in infants: recommendations of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb Mortal Wkly Rep 2023;72:1115–22. 10.15585/mmwr.mm7241e1 - DOI - PMC - PubMed
-
- CDC. Emergency preparedness and response: limited availability of nirsevimab in the United States—interim CDC recommendations to protect infants from respiratory syncytial virus (RSV) during the 2023–2024 respiratory virus season. Atlanta, GA: US Department of Health and Human Services, CDC; 2023. https://www.cdc.gov/han/2023/han00499.html
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

