Fatty acid metabolism suppresses neonatal cardiomyocyte proliferation by increasing PDK4 and HMGCS2 expression through PPARδ
- PMID: 40338840
- PMCID: PMC12061097
- DOI: 10.1371/journal.pone.0318178
Fatty acid metabolism suppresses neonatal cardiomyocyte proliferation by increasing PDK4 and HMGCS2 expression through PPARδ
Abstract
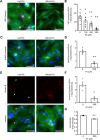
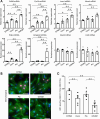
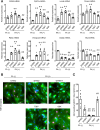
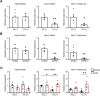
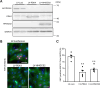
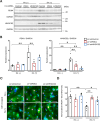
Cardiomyocytes lose their capacity to regenerate immediately after birth. Simultaneously, cardiomyocytes change energy metabolism from glycolysis to oxidative phosphorylation, especially using fatty acids. Accumulating evidence has revealed that fatty acid metabolism weakens the proliferative ability of cardiomyocytes. However, its underlying molecular mechanism remains unclear. In this study, we investigated how fatty acid metabolism contributes to cell cycle regulation in neonatal cardiomyocytes. Cultured neonatal rat cardiomyocytes (NRCMs) were treated with a fatty acid mixture (FA) consisting of palmitic and oleic acids containing L-carnitine. The FA treatment increased not only β-oxidation-related enzymes but also pyruvate dehydrogenase kinase 4 (PDK4), a fatty acid metabolism regulator, and HMG-CoA synthase 2 (HMGCS2), a ketogenic factor. Moreover, Ki67-positive proliferative NRCMs were reduced by the FA, indicating that fatty acids suppress the NRCM cell cycle. GW501516, a peroxisome proliferator-activated receptor δ (PPARδ) activator, also upregulated fatty acid metabolism genes and disturbed NRCM proliferation, whereas GSK3787, a PPARδ inhibitor, recovered FA-induced the cell cycle arrest. Furthermore, overexpression of PDK4 or HMGCS2 using a lentiviral vector suppressed cell cycle activity in NRCMs, and silencing either gene regained cell cycle even in FA-rich condition. In conclusion, fatty acid metabolism increased PDK4 and HMGCS2 via PPARδ activation and suppressed NRCM proliferation.
Copyright: © 2025 Tanaka et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have no conflicts of interest associated with this manuscript.
Figures

Similar articles
-
Peroxisome proliferator-activated receptor delta activates fatty acid oxidation in cultured neonatal and adult cardiomyocytes.Biochem Biophys Res Commun. 2004 Jan 9;313(2):277-86. doi: 10.1016/j.bbrc.2003.11.127. Biochem Biophys Res Commun. 2004. PMID: 14684157
-
High Fat Diet Upregulates Fatty Acid Oxidation and Ketogenesis via Intervention of PPAR-γ.Cell Physiol Biochem. 2018;48(3):1317-1331. doi: 10.1159/000492091. Epub 2018 Jul 26. Cell Physiol Biochem. 2018. PMID: 30048968 Free PMC article.
-
CD36-dependent regulation of muscle FoxO1 and PDK4 in the PPAR delta/beta-mediated adaptation to metabolic stress.J Biol Chem. 2008 May 23;283(21):14317-26. doi: 10.1074/jbc.M706478200. Epub 2008 Feb 28. J Biol Chem. 2008. PMID: 18308721 Free PMC article.
-
Regulation of energy metabolism by long-chain fatty acids.Prog Lipid Res. 2014 Jan;53:124-44. doi: 10.1016/j.plipres.2013.12.001. Epub 2013 Dec 18. Prog Lipid Res. 2014. PMID: 24362249 Review.
-
Metabolic Reprogramming: A Byproduct or a Driver of Cardiomyocyte Proliferation?Circulation. 2024 May 14;149(20):1598-1610. doi: 10.1161/CIRCULATIONAHA.123.065880. Epub 2024 May 13. Circulation. 2024. PMID: 38739695 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

