Anticoagulant prescribing trends, bleeding events, and reversal agent use in pediatric patients: A retrospective, real-world study
- PMID: 40338907
- PMCID: PMC12061172
- DOI: 10.1371/journal.pone.0323137
Anticoagulant prescribing trends, bleeding events, and reversal agent use in pediatric patients: A retrospective, real-world study
Abstract
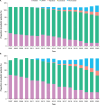
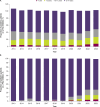
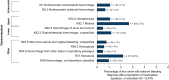
This retrospective real-world study aimed to describe anticoagulant prescribing trends, particularly for factor Xa (FXa) inhibitors, bleeding events, and reversal agent use in pediatric patients to assess potential populations for clinical trials of the FXa inhibitor reversal agent andexanet alfa. Real-world health care data from the TriNetX Global Network and Optum's deidentified Clinformatics® Data Mart Database (CDM) were analyzed to identify patients aged <18 years old who were prescribed a direct oral FXa inhibitor, warfarin, or low-molecular-weight heparins from 2007 through 2024 (TriNetX, N = 59,780) or 2023 (CDM, N = 6470). The only anticoagulants prescribed to children were warfarin and/or low-molecular-weight heparins in 2007 and 2008 in TriNetX and from 2007 through 2010 in CDM. Prescriptions of the FXa inhibitor rivaroxaban increased from 0.4% (2009) to 18.0% (2023) in TriNetX and from 0.8% (2011) to 34.0% (2023) in CDM, with similar trends for apixaban. Relevant bleeding was reported in 9.4% of patients prescribed an FXa inhibitor in TriNetX; ≤ 0.1% of patients received andexanet alfa the day of a bleed. Among patients prescribed an FXa inhibitor, ≤ 0.1% in TriNetX and 0 in CDM received andexanet alfa the day of surgery. Direct oral FXa inhibitor use in children is growing, as is the potential for associated bleeds; however, reversal agent use is rare in this population. Given the possible unmet need and subsequent patient recruitment challenges, designing pediatric clinical trials of reversal agents requires innovative approaches.
Copyright: © 2025 D’Abrantes et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
SD, MB, PA, AB, and UM are employees of AstraZeneca and own stock options. CM reports fees to his institution for study participation from Bayer, Bristol Myers Squibb, and Pfizer; and personal honoraria from Anthos, AstraZeneca, Bayer, Chiesi, Janssen, and Norgine. NB has no conflicts of interest to declare. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- Jaffray J, Branchford B, Goldenberg N, Malvar J, Croteau SE, Silvey M, et al. Development of a risk model for pediatric hospital-acquired thrombosis: a report from the children’s hospital-acquired thrombosis consortium. J Pediatr. 2021;228:252–259.e1. doi: 10.1016/j.jpeds.2020.09.016 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

