Gene delivery of AGAT and GAMT boosts creatine levels in creatine transporter deficiency patient fibroblasts
- PMID: 40338959
- PMCID: PMC12061113
- DOI: 10.1371/journal.pone.0319350
Gene delivery of AGAT and GAMT boosts creatine levels in creatine transporter deficiency patient fibroblasts
Abstract
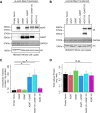
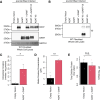
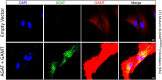
Creatine is a critical metabolite used to buffer cellular energy demands in highly energetic tissues such as the brain and muscle. Genetic defects in endogenous creatine synthesis or transport across cellular membranes lead to a common set of phenotypes referred to as Cerebral Creatine Deficiency Syndrome (CCDS). The most common form of CCDS is Creatine Transporter 1 (CT1) Deficiency (CTD). It accounts for ~ 70% of cases and results from loss-of-function mutations in the X-linked gene SLC6A8. Affected individuals suffer from intellectual disability, autistic-like behaviors, and epilepsy. There are currently no effective therapies for this disorder, but gene therapy has emerged as a potential approach. The two enzymes which comprise the endogenous creatine synthetic pathway (AGAT and GAMT) are selectively expressed by specific cell types throughout the body. However, after synthesized, creatine uptake relies on the protein product of SLC6A8, CT1, to transport creatine into target cell types. We hypothesized that gene delivery of GATM (encoding AGAT) and GAMT into end-user cell types would bypass the need for CT1, allowing for intracellular synthesis of creatine. We tested this strategy in two human cell types: HEK293T cells and primary fibroblasts. Co-delivery of GATM and GAMT increased internal creatine concentrations by 7.6-fold in HEK293T cells and 12.3-fold in healthy control fibroblasts. We then employed this approach to primary fibroblasts from patients with CTD. This resulted in an up to 11.6-fold increase in intracellular creatine concentrations, far exceeding the intracellular concentration of creatine in healthy control fibroblasts. Importantly, overexpression of AGAT and GAMT resulted in proper targeting of these enzymes to their natural cellular compartment and did not impair the growth of patient fibroblasts. These findings establish gene therapy with GATM and GAMT as a potential strategy for patients with CTD.
Copyright: © 2025 Wells et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: JR is a founder of Vettore Biosciences and a member of its scientific advisory board. All other authors declare no competing interests.
Figures




Similar articles
-
ClinGen variant curation expert panel recommendations for classification of variants in GAMT, GATM and SLC6A8 for cerebral creatine deficiency syndromes.Mol Genet Metab. 2024 May;142(1):108362. doi: 10.1016/j.ymgme.2024.108362. Epub 2024 Mar 2. Mol Genet Metab. 2024. PMID: 38452609 Free PMC article.
-
Creatine biosynthesis and transport in health and disease.Biochimie. 2015 Dec;119:146-65. doi: 10.1016/j.biochi.2015.10.022. Epub 2015 Nov 2. Biochimie. 2015. PMID: 26542286 Review.
-
Dissociation of AGAT, GAMT and SLC6A8 in CNS: relevance to creatine deficiency syndromes.Neurobiol Dis. 2010 Feb;37(2):423-33. doi: 10.1016/j.nbd.2009.10.022. Epub 2009 Oct 29. Neurobiol Dis. 2010. PMID: 19879361
-
Creatine synthesis and exchanges between brain cells: What can be learned from human creatine deficiencies and various experimental models?Amino Acids. 2016 Aug;48(8):1877-95. doi: 10.1007/s00726-016-2189-0. Epub 2016 Feb 10. Amino Acids. 2016. PMID: 26861125 Review.
-
Biochemical, molecular, and clinical diagnoses of patients with cerebral creatine deficiency syndromes.Mol Genet Metab. 2013 Jul;109(3):260-8. doi: 10.1016/j.ymgme.2013.04.006. Epub 2013 Apr 17. Mol Genet Metab. 2013. PMID: 23660394
References
-
- Mercimek-Andrews S, Salomons GS. Creatine Deficiency Syndromes. University of Washington, Seattle, Seattle (WA); 2022. Available: http://europepmc.org/abstract/MED/20301745 - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

