Unveiling the intercompartmental signaling axis: Mitochondrial to ER Stress Response (MERSR) and its impact on proteostasis
- PMID: 40338975
- PMCID: PMC12088515
- DOI: 10.1371/journal.pgen.1011700
Unveiling the intercompartmental signaling axis: Mitochondrial to ER Stress Response (MERSR) and its impact on proteostasis
Abstract
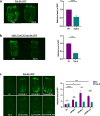
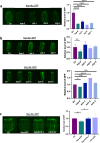
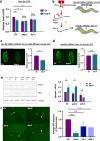
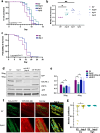
Maintaining protein homeostasis is essential for cellular health. Our previous research uncovered a cross-compartmental Mitochondrial to Cytosolic Stress Response, activated by the perturbation of mitochondrial proteostasis, which ultimately results in the improvement of proteostasis in the cytosol. Here, we found that this signaling axis also influences the unfolded protein response of the endoplasmic reticulum (UPRER), suggesting the presence of a Mitochondria to ER Stress Response (MERSR). During MERSR, the IRE1 branch of UPRER is inhibited, introducing a previously unknown regulatory component of MCSR. Moreover, proteostasis is enhanced through the upregulation of the PERK-eIF2α signaling pathway, increasing phosphorylation of eIF2α and improving the ER's ability to handle proteostasis. MERSR activation in both polyglutamine and amyloid-beta peptide-expressing C. elegans disease models also led to improvement in both aggregate burden and overall disease outcome. These findings shed light on the coordination between the mitochondria and the ER in maintaining cellular proteostasis and provide further evidence for the importance of intercompartmental signaling.
Copyright: © 2025 Li et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Update of
-
Unveiling the Intercompartmental Signaling Axis: Mitochondrial to ER Stress Response (MERSR) and its Impact on Proteostasis.bioRxiv [Preprint]. 2024 Sep 14:2023.09.07.556674. doi: 10.1101/2023.09.07.556674. bioRxiv. 2024. Update in: PLoS Genet. 2025 May 8;21(5):e1011700. doi: 10.1371/journal.pgen.1011700. PMID: 38187690 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

