Hyperactivation of RAB5 disrupts the endosomal Rab cascade leading to endolysosomal dysregulation in Down syndrome: A necessary role for increased APP gene dose
- PMID: 40339170
- PMCID: PMC12058456
- DOI: 10.1002/alz.70046
Hyperactivation of RAB5 disrupts the endosomal Rab cascade leading to endolysosomal dysregulation in Down syndrome: A necessary role for increased APP gene dose
Abstract
Introduction: Down syndrome (DS) markedly increases the risk of Alzheimer's disease (DS-AD), but the role of RAB5 hyperactivation in its pathogenesis remains unclear.
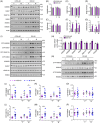
Methods: Postmortem brain samples from individuals with DS, with and without AD, and a partial trisomy 21 case with only two amyloid precursor protein (APP) gene copies, were examined for endosomal Rabs, their guanine-nucleotide exchange factor (GEF) and GTPase activating protein (GAP) levels, and lysosomal cathepsins. Analysis extended to the Dp16 DS mouse model. The role of RAB5 hyperactivation in disrupting the endolysosomal system was explored using primary neurons.
Results: We observed widespread endolysosomal dysregulation in DS and Dp16 brains, requiring increased APP gene dose. RAB5 hyperactivation resulted in increased activation of endosomal Rabs, including RABs 7 and 11, and increased recruitment of Rabs and their GEFs to early endosomes as well as the levels of lysosomal cathepsins.
Discussion: These findings suggest that APP dose-driven RAB5 hyperactivation disrupts endosomal Rab cascades and endosome maturation in DS.
Highlights: There is widespread disruption of the endolysosomal network in the Down syndrome (DS) brain and in the Dp16 mouse model brain. Amyloid precursor protein (APP) gene dose was necessary for increases in endosomal Rab activity and lysosomal cathepsins in both human and mouse brains. Changes in endosomal Rabs 7 and 11 were linked to increases in their guanine-nucleotide exchange factors (GEFs) and GEF/GTPase activating protein (GAP) ratios. Mechanistic studies demonstrated essential roles for the beta-C-terminal fragment (β-CTF) of APP acting through hyperactivation of RAB5 to increase early endosomal membrane binding of the GEFs for downstream endosomal Rabs. RAB5 acts as the central hub for disruptions in endolysosomal function in DS.
Keywords: APP; Alzheimer's disease; Dp16 mouse; GEF; RAB5; cathepsin; down syndrome; endosomal rab cascade.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
W.C.M. serves as a Scientific Advisory Board (SAB) member and holds stock options from Alzheon, Inc. and Promis, Inc. W.C.M. also serves as an SAB member and holds stock in Acta Pharmaceuticals, Inc. His name is on a patent under University of California San Diego and Massachusetts General Hospital concerning γ‐secretase modulators licensed to Acta Pharmaceuticals, Inc. He has served as a consultant to AC Immune. W.C.M. holds a leadership position in the Trisomy 21 Research Society. He serves on committees for the Alzheimer's Project San Diego and the American Neurological Association and an NIH COBRE Grant to the University of Nebraska. W.C.M. received a royalty payment under a patent held by Stanford University licensed to Curasen. Author disclosures are available in the Supporting Information.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

