CalliCog is an open-source cognitive neuroscience toolkit for freely behaving nonhuman primates
- PMID: 40339574
- PMCID: PMC12146646
- DOI: 10.1016/j.crmeth.2025.101034
CalliCog is an open-source cognitive neuroscience toolkit for freely behaving nonhuman primates
Abstract
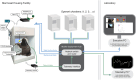
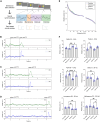
Nonhuman primates (NHPs) are pivotal for unlocking the complexities of human cognition, yet traditional cognitive studies remain constrained to specialized laboratories. To address this gap, we present CalliCog: an open-source, scalable in-cage platform tailored for experiments in small freely behaving primate species such as the common marmoset (Callithrix jacchus). CalliCog includes modular operant chambers that operate autonomously and integrate seamlessly with home cages, eliminating human intervention. Our results showcase the power of CalliCog to train experimentally naive marmosets in touchscreen-based cognitive tasks. Across two independent facilities, marmosets achieved touchscreen proficiency within 2 weeks and successfully completed tasks probing behavioral flexibility and working memory. Moreover, CalliCog enabled precise synchronization of behavioral data with electrocorticography (ECoG) recordings from freely moving animals, opening new frontiers for neurobehavioral research. By making CalliCog openly accessible, we aim to democratize cognitive experimentation with small NHPs, narrowing the translational gap between preclinical models and human cognition.
Keywords: CP: Biotechnology; CP: Neuroscience; automation; behavior; behavioral flexibility; cognition; electrocorticography; marmoset; operant chamber; positive reinforcement; prefrontal cortex; working memory.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




Update of
-
CalliCog: an open-source cognitive neuroscience toolkit for freely behaving nonhuman primates.bioRxiv [Preprint]. 2024 Nov 26:2024.11.26.625450. doi: 10.1101/2024.11.26.625450. bioRxiv. 2024. Update in: Cell Rep Methods. 2025 May 19;5(5):101034. doi: 10.1016/j.crmeth.2025.101034. PMID: 39651268 Free PMC article. Updated. Preprint.
References
-
- Kondo T., Saito R., Otaka M., Yoshino-Saito K., Yamanaka A., Yamamori T., Watakabe A., Mizukami H., Schnitzer M.J., Tanaka K.F., et al. Calcium Transient Dynamics of Neural Ensembles in the Primary Motor Cortex of Naturally Behaving Monkeys. Cell Rep. 2018;24:2191–2195.e4. doi: 10.1016/j.celrep.2018.07.057. - DOI - PubMed
-
- Trautmann E.M., Hesse J.K., Stine G.M., Xia R., Zhu S., O’Shea D.J., Karsh B., Colonell J., Lanfranchi F.F., Vyas S., et al. Large-scale high-density brain-wide neural recording in nonhuman primates. bioRxiv. 2023 doi: 10.1101/2023.02.01.526664. Preprint at. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

