Chloroplast ATP-dependent metalloprotease FtsH5/VAR1 confers cold-stress tolerance through singlet oxygen and salicylic acid signaling
- PMID: 40340256
- PMCID: PMC12177481
- DOI: 10.1016/j.xplc.2025.101353
Chloroplast ATP-dependent metalloprotease FtsH5/VAR1 confers cold-stress tolerance through singlet oxygen and salicylic acid signaling
Abstract
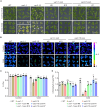
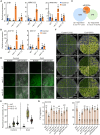
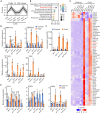
Cold stress substantially affects plant growth and productivity. Chloroplasts are primary sites for the production of reactive oxygen species (ROS) and biosynthesis of the defense hormone salicylic acid (SA) under environmental stress conditions. However, the mechanisms by which plants integrate ROS and SA signaling to adapt to stress remain elusive. Here, we report that Arabidopsis FILAMENTOUS TEMPERATURE-SENSITIVE H5/YELLOW VARIEGATED1 (FtsH5/VAR1), a thylakoid-localized ATP-dependent zinc metalloprotease, is essential for plant cold-stress tolerance. The var1-1 mutant exhibits pronounced chlorosis and variegation, as well as retarded growth under cold stress conditions. We observed a strong correlation between elevated SA biosynthesis/signaling and the cold-sensitive phenotype of var1. Reducing SA accumulation, either by overexpressing the salicylate hydroxylase gene (NahG) or knocking out SA biosynthesis-related genes (ICS1, EDS1, or PAD4), partially suppressed the chlorosis phenotype of var1. Furthermore, we demonstrated that EXECUTOR1 (EX1)-mediated singlet oxygen (1O2) signaling acts upstream of EDS1 to regulate the expression of SA-responsive genes (SARGs) in var1 under cold stress. Notably, we identified a critical role for EX2, in which the mutation in EX2 significantly suppressed the cold-sensitive phenotype of var1, in activating the expression of SARGs while repressing photosynthesis-associated nuclear genes. Collectively, our results suggest a vital role for VAR1 in plant cold tolerance and highlight the tight connection between 1O2 and SA signaling, elucidating a previously unheeded function of EX2, which likely operates independently of EX1-mediated 1O2 signaling.
Keywords: EX2; VAR1; cold stress; salicylic acid; singlet oxygen.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures




Similar articles
-
EXECUTER1 and singlet oxygen signaling: A reassessment of nuclear activity.Plant Cell. 2024 Dec 23;37(1):koae296. doi: 10.1093/plcell/koae296. Plant Cell. 2024. PMID: 39499663
-
The chloroplast singlet oxygen-triggered biosynthesis of salicylic acid and jasmonic acid is mediated by EX1 and GUN1 in Arabidopsis.Plant Cell Environ. 2024 Aug;47(8):2852-2864. doi: 10.1111/pce.14910. Epub 2024 Apr 10. Plant Cell Environ. 2024. PMID: 38600785
-
The Arabidopsis chloroplast protein HHL1 regulates AvrRpt2-triggered immunity via light-dependent reactive oxygen species homeostasis.J Integr Plant Biol. 2025 Aug;67(8):2151-2166. doi: 10.1111/jipb.13929. Epub 2025 May 12. J Integr Plant Biol. 2025. PMID: 40353630
-
The interplay of singlet oxygen and ABI4 in plant growth regulation.Trends Plant Sci. 2025 Feb;30(2):156-166. doi: 10.1016/j.tplants.2024.09.007. Epub 2024 Oct 15. Trends Plant Sci. 2025. PMID: 39414457 Review.
-
Salicylic acid in plant immunity and beyond.Plant Cell. 2024 May 1;36(5):1451-1464. doi: 10.1093/plcell/koad329. Plant Cell. 2024. PMID: 38163634 Free PMC article. Review.
Cited by
-
Molecular Networks Governing Plant Responses to Heat and Cold Stress.Plants (Basel). 2025 Jul 7;14(13):2073. doi: 10.3390/plants14132073. Plants (Basel). 2025. PMID: 40648082 Free PMC article. Review.
References
-
- Bartsch M., Gobbato E., Bednarek P., Debey S., Schultze J.L., Bautor J., Parker J.E. Salicylic acid-independent ENHANCED DISEASE SUSCEPTIBILITY1 signaling in Arabidopsis immunity and cell death is regulated by the monooxygenase FMO1 and the Nudix hydrolase NUDT7. Plant Cell. 2006;18:1038–1051. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

