Systematic Engineering for Efficient Uric Acid-Degrading Activity in Probiotic Yeast Saccharomyces boulardii
- PMID: 40340401
- PMCID: PMC12186688
- DOI: 10.1021/acssynbio.4c00831
Systematic Engineering for Efficient Uric Acid-Degrading Activity in Probiotic Yeast Saccharomyces boulardii
Abstract
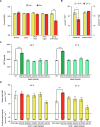
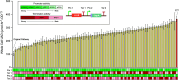
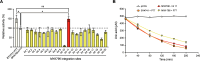
Hyperuricemia, caused by uric acid disequilibrium, is a prevalent metabolic disease that most commonly manifests as gout and is closely associated with a spectrum of other comorbidities such as renal disorders and cardiovascular diseases. While natural and engineered probiotics that promote catabolism of uric acid in the intestine have shown promise in relieving hyperuricemia, limitations in strain efficiency and the requirements for achieving high performance remain major hurdles in the practical application of probiotic-mediated prevention and management. Here, we employed a systematic strategy to engineer a high-efficiency uric acid catabolism pathway in S. cerevisiae. An uricase from Vibrio vulnificus, exhibiting high-level activity in S. cerevisiae, was identified as the uric acid-degrading component. The expression level and stability of urate transporter UapA were improved by constructing a chimera, enabling reliable uric acid import in S. cerevisiae. Additionally, constitutive promoters were selected and combinatorially assembled with the two functional components, creating a collection of pathways that confer varied levels of uric acid catabolic activity to S. cerevisiae. The best-performing pathway can express uric acid-degrading activity up to 365.32 ± 20.54 μmol/h/OD, requiring only simple cultivation steps. Eventually, we took advantage of the genetic similarity between model organism S. cerevisiae and probiotic S. boulardii and integrated the optimized pathway into identified high-expression integration loci in the S. boulardii genome. The activity can be stably maintained under high-density fermentation conditions. Overall, this study provided a high-potential hyperuricemia-managing yeast probiotic strain, demonstrating the capabilities of developing recombinant probiotics.
Keywords: Saccharomyces boulardii; engineered probiotic; hyperuricemia; urate transporter; uric acid degradation; uricase.
Figures

Similar articles
-
In Situ Biomanufacturing of Small Molecules in the Mammalian Gut by Probiotic Saccharomyces boulardii.ACS Synth Biol. 2021 May 21;10(5):1039-1052. doi: 10.1021/acssynbio.0c00562. Epub 2021 Apr 12. ACS Synth Biol. 2021. PMID: 33843197
-
Dietary interventions for recurrent abdominal pain in childhood.Cochrane Database Syst Rev. 2017 Mar 23;3(3):CD010972. doi: 10.1002/14651858.CD010972.pub2. Cochrane Database Syst Rev. 2017. PMID: 28334433 Free PMC article.
-
Genetic engineering of Saccharomyces boulardii: Tools, strategies and advances for enhanced probiotic and therapeutic applications.Biotechnol Adv. 2025 Jul 30;84:108663. doi: 10.1016/j.biotechadv.2025.108663. Online ahead of print. Biotechnol Adv. 2025. PMID: 40750061 Review.
-
Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children.Cochrane Database Syst Rev. 2017 Dec 19;12(12):CD006095. doi: 10.1002/14651858.CD006095.pub4. Cochrane Database Syst Rev. 2017. PMID: 29257353 Free PMC article.
-
Urate oxidase for the prevention and treatment of tumour lysis syndrome in children with cancer.Cochrane Database Syst Rev. 2017 Mar 8;3(3):CD006945. doi: 10.1002/14651858.CD006945.pub4. Cochrane Database Syst Rev. 2017. PMID: 28272834 Free PMC article.
References
-
- Gill D., Cameron A. C., Burgess S., Li X., Doherty D. J., Karhunen V., Abdul-Rahim A. H., Taylor-Rowan M., Zuber V., Tsao P. S., Klarin D., Evangelou E., Elliott P., Damrauer S. M., Quinn T. J., Dehghan A., Theodoratou E., Dawson J., Tzoulaki I.. Blood Pressure, and Cardiovascular Disease: Evidence From Mendelian Randomization and Meta-Analysis of Clinical Trials. Hypertension. 2021;77(2):383–392. doi: 10.1161/HYPERTENSIONAHA.120.16547. - DOI - PMC - PubMed
-
- Li X., Meng X., Timofeeva M., Tzoulaki I., Tsilidis K. K., Ioannidis J. P., Campbell H., Theodoratou E.. Serum Uric Acid Levels and Multiple Health Outcomes: Umbrella Review of Evidence from Observational Studies, Randomised Controlled Trials, and Mendelian Randomisation Studies. BMJ. 2017;357:j2376. doi: 10.1136/bmj.j2376. - DOI - PMC - PubMed
-
- Tu C.-M., Wei T.-E., Tseng G.-S., Chen C.-C., Liu C.-W.. Serum Uric Acid Is Associated with Incident Metabolic Syndrome Independent of Body Shape Index and Body Roundness Index in Healthy Individuals. Nutr Metab Cardiovasc Dis. 2021;31(11):3142–3151. doi: 10.1016/j.numecd.2021.07.008. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

