Peripheral blood cytokine profiles predict the severity of SARS-CoV-2 infection: an EPIC3 study analysis
- PMID: 40340594
- PMCID: PMC12063214
- DOI: 10.1186/s12879-025-10914-6
Peripheral blood cytokine profiles predict the severity of SARS-CoV-2 infection: an EPIC3 study analysis
Abstract
Background: Predicting which patients will develop severe COVID-19 complications could improve clinical care. Peripheral blood cytokine profiles may predict the severity of SARS-CoV-2 infection, but none have been identified in US Veterans.
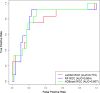
Methods: We analyzed peripheral blood cytokine profiles from 202 participants in the EPIC3 study, a prospective observational cohort of US Veterans tested for SARS-CoV-2 across 15 VA medical centers. Illness severity was assessed based on the highest level documented during the first 60 days after recruitment. We correlated cytokine levels with illness severity using LASSO logistic regression, random forest, and XGBoost models on a 70% training set and calculated the AUC on a 30% test set.
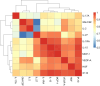
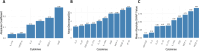
Results: LASSO regression identified 6 cytokines as predictors of SARS-CoV-2 severity with 77.3% AUC in the test set. Random forest and XGBoost models achieved an AUC of 80.4% and 80.7% in the test set, respectively. All models assigned a feature importance to each cytokine, with IP-10, MCP-1, and HGF consistently identified as key markers.
Conclusions: Cytokine profiles are predictive of SARS-CoV-2 severity in US Veterans and may guide tailored interventions for improved patient management.
Keywords: COVID-19; Cytokines; Illness severity; LASSO regression; Predictive biomarkers; Random forest; SARS-CoV-2; Veterans; XGBoost.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All study participants provided their individual written, informed consent to participate in the EPIC3 study. Our study is approved by the VA Central Institutional Review Board (reference 20–14). Consent for publication: The authors and participants provided their consent to have this manuscript and the data it contains published. Competing interests: The authors declare no competing interests.
Figures
References
-
- (CDC) CfDCaP. COVID Data Tracker. Accessed Aug 31, 2024. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous