The most bothersome symptoms in neuromuscular diseases: the ERN EURO NMD Survey
- PMID: 40340786
- PMCID: PMC12063438
- DOI: 10.1186/s13023-025-03742-z
The most bothersome symptoms in neuromuscular diseases: the ERN EURO NMD Survey
Abstract
Background: Neuromuscular diseases (NMDs) comprise a range of genetic and acquired rare disorders that affect motor neurons, peripheral nerves, neuromuscular junctions and skeletal muscles, leading to significant impairments such as muscle weakness and fatigue resulting in functional limitations. This study aims to investigate the prevalence and severity of disease-related symptoms in adult patients with NMDs registered in the European Reference Network (ERN) EURO-NMD. A cross-sectional electronic survey was conducted with 1,253 participants who reported the severity of 28 symptoms, which were scored using multi-criteria decision analysis (MCDA).
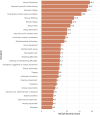
Results: The results identified muscle fatigue, weakness and impaired physical function/activity as the most severe and prevalent symptoms in all NMD groups, followed by coordination and/or balance problems, muscle stiffness, mental fatigue, and pain. Notably, the analysis highlighted differences in symptom severity between disease subtypes and underlined the need for standardised patient-reported outcome measures (PROMs) to address the broad heterogeneity of NMDs.
Conclusions: The findings stress the critical importance of capturing patient perspectives to guide clinical care, research priorities and therapeutic development. This work argues for the development of uniform PROMs to better assess disease impact, natural history and treatment efficacy, contributing to improved patient-centred care across diverse NMD populations.
Keywords: Neuromuscular diseases; PROMs; Symptoms.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study did not require research ethics committee approval because it involves secondary analysis of anonymized aggregate data that adheres to recognized anonymization standards and does not contain sensitive information (e.g., clinical or genetic data). The study complies with all applicable ethical guidelines and data protection regulations. Additionally, it was conducted in accordance with the Helsinki Declaration of 1964, its subsequent amendments, and equivalent ethical standards where applicable. Consent for publication: Not applicable. Competing interests: MM has received honoraria for speaking, advisory boards and compensation for congress participation from Sanofi Genzyme, Khondrion, Biogen, UCB, Stealth and Precision Biosciences, outside the submitted work. LM has received honoraria for speaking, advisory boards and compensation for congress participation from Sanofi Genzyme, Roche, Biogen, Amicus Therapeutics, Alexion, Argenx, UCB, Janssen, Lupin, outside the submitted work. PV received honoraria, consultation fees, and compensation for advisory board participation from Pfizer, Sanofi Genzyme, CSL Behring, LFB, Natus, UCB Pharma, Janssen, Alnylam. VS received compensation for consulting services and/or speaking activities from AveXis, Cytokinetics, Italfarmaco, LiquidwebS.r.l., Novartis Pharma AG, Amylyx Pharmaceuticals, Biogen, and Zambon Biotech SA. MdV is a consultant to Novartis, Argenx and Astra Zeneca. CK acknowledges participation in Advisory Boards of Amicus Therapeutics, Chiesi, Fulcrum Therapeutics, Hormosan, Roche Pharma AG, Sanofi Genzyme, Stealth BioTherapeutics, and participation as local PI in clinical trials by Amicus Therapeutics, Fulcrum Therapeutics, Omeicos, Reneo Pharmaceuticals, Stealth Therapeutics, Scholar Rocks, and received travel grants and/or lecture fees from Amicus Therapeutics, Chiesi, Sanofi Genzyme, Argenx, Novartis, Santhera, Sarepta, UCB. DP acknowledges participation in Advisory Boards of Inflectis, Alnylam, Akcea, Arvinas, Augustine Tx, DTx, Novartis, participation as local PI in clinical trials sponsored by Alnylam, Ionis, AT Therapeutics, Nido-Biosciences. The remaining authors declare that they have no competing interests.
Figures



References
-
- Rose L, McKim D, Leasa D, Nonoyama M, Tandon A, Bai YQ et al. Trends in incidence, prevalence, and mortality of neuromuscular disease in Ontario, Canada: A population-based retrospective cohort study (2003–2014). PLoS One. 2019 Mar 1 [cited 2025 Mar 8];14(3). Available from: https://pubmed.ncbi.nlm.nih.gov/30913206/ - PMC - PubMed
-
- Graham CD, Rose MR, Grunfeld EA, Kyle SD, Weinman J. A systematic review of quality of life in adults with muscle disease. J Neurol. 2011 [cited 2025 Mar 8];258(9):1581–92. Available from: https://pubmed.ncbi.nlm.nih.gov/21597956/ - PubMed
-
- Zizzi C, Seabury J, Rosero S, Alexandrou D, Wagner E, Weinstein JS et al. Patient reported impact of symptoms in amyotrophic lateral sclerosis (PRISM-ALS): A national, cross-sectional study. EClinicalMedicine. 2022 Jan 1 [cited 2025 Mar 8];55. Available from: https://pubmed.ncbi.nlm.nih.gov/36531982/ - PMC - PubMed
-
- Thomas FP, Saporta MA, Attarian S, Sevilla T, Sivera R, Fabrizi GM et al. Patient-Reported Symptom Burden of Charcot–Marie–Tooth Disease Type 1A: Findings From an Observational Digital Lifestyle Study. J Clin Neuromuscul Dis. 2022 Sep 1 [cited 2025 Mar 8];24(1):7–17. Available from: https://journals.lww.com/10.1097/CND.0000000000000426 - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

