M64HCl, a focal adhesion kinase activator, promotes intestinal mucosal healing in rats
- PMID: 40340802
- PMCID: PMC12063223
- DOI: 10.1186/s12876-025-03937-5
M64HCl, a focal adhesion kinase activator, promotes intestinal mucosal healing in rats
Abstract
Background: Intestinal mucosal injury may arise from various factors. While many drugs target the causative factors, none directly stimulate mucosal wound healing. We found that the specific focal adhesion kinase (FAK) activator, M64HCl, promotes intestinal mucosal healing in mice. This study aims to further validate the therapeutic impact of M64HCl on intestinal mucosal repair in rats as a second species.
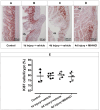
Methods: Wistar rats were assigned to one of four groups: normal control, 1-day injury + vehicle, 4-day injury + vehicle, or 4-day injury + M64HCl. Intestinal injury was induced by serosally applying 75% acetic acid. Immediately after injury, rats received either a continuous infusion of M64HCl (25 mg/kg/day) or its vehicle (saline). Four days post-injury, blood was drawn to measure M64HCl levels and assess liver and kidney function. The intestines were removed and opened, ulcer areas were photographed for size quantification, and tissues were fixed for histological and immunohistochemical analysis.
Results: M64HCl substantially reduced ulcer area on gross examination, while histological analysis showed alleviation of pathological changes with M64HCl treatment. Immunohistochemical analysis confirmed increased immunoreactivity for phosphorylated FAK in the epithelium adjacent to the injury in M64HCl-treated rats. However, there was no change in the percentage of Ki67-positive cells in each crypt at the edge of the ulcer area. Serum creatinine, ALT, and AST levels did not differ between the 4-day injury groups with or without M64HCl treatment.
Conclusions: M64HCl, a water-soluble FAK activator, promotes acetic acid-induced ulcer healing in rats and may be useful in treating gastrointestinal mucosal injury.
Keywords: Focal adhesion kinase; Mucosal healing; Small intestine, mucosal injury.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee (IACUC) of Northeast Ohio Medical University, with IACUC number 23-04-366. The experiments were conducted in accordance with relevant guidelines and regulations. Consent for publication: Not applicable. Competing interests: The University of North Dakota and the University of Minnesota have filed two patent applications on the use of small-molecule FAK activators to promote mucosal healing. MDB and VJG are listed as co-inventors, and one also includes RGM.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

