The glycosaminoglycan chains of perlecan regulate the perivascular fluid transport
- PMID: 40340918
- PMCID: PMC12063283
- DOI: 10.1186/s12987-025-00648-7
The glycosaminoglycan chains of perlecan regulate the perivascular fluid transport
Abstract
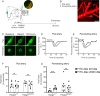
Background: The perivascular conduct pathway that connects the cerebrospinal fluid spaces with the interstitial fluid in the parenchyma are of importance for solute clearance from the brain. In this pathway, the relatively wide perivascular space (PVS) surrounding the pial arteries provides a low-resistant passage while around the perforating arteries, the solute movement is along the basement membrane (BM), that prevents the free exchange of interstitial fluids and solutes. We hypothesize that this selectivity involves specific components of the vascular BM, which is mainly composed of type IV collagen (Col IV) and laminin networks interconnected by nidogens and heparan sulphate proteoglycans (HSPGs). Perlecan is the major HSPG in the BM that binds to Col IV and laminin via glycosaminoglycan (GAG) chains to form a molecular sieve. GAGs may also provide the charge selectivity required for filtration, and also a scaffold for amyloid-β (Aβ) aggregation. The purpose of this study was the functional characterization of perivascular fluid transport and brain clearance in mice lacking perlecan GAG chains.
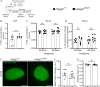
Methods: We generated a novel mouse line (Hspg2∆3∆91) lacking perlecan GAG side chains and investigated perivascular flow and brain clearance in these mice using intravital multiphoton and fluorescence recovery after photobleaching techniques, and functional assays with various tracers. Potentially deleterious effects on brain homeostasis were investigated using transcriptomic, proteomic and immunohistochemical methods. The Hspg2∆3∆91 mice were crossed with a 5xFAD line to examine the importance of GAGs in Aβ aggregation.
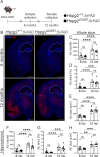
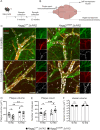
Results: We observed a delayed inflow of CSF tracer into the Hspg2∆3∆91 brain with no changes in the clearance of parenchymal injected tracers. Quantification of the Aβ plaques revealed fewer and smaller plaques in the walls of the pial arteries at six months of age, but not in the brain parenchyma. Surprisingly, perlecan GAG deficiency had no severe deleterious effects on brain homeostasis in transcriptomic and proteomic analyses.
Conclusions: Potential brain clearance mechanisms are dependent on the flow through special ECM structures. BM is mainly known for its barrier function, whereas very little is known about how passage along the perivascular ECM is established. This study shows that the GAG composition of the BM affects the solute dynamics and Aβ deposition in the periarterial space.
Keywords: Amyloid beta; Basement membrane; Cerebrospinal fluid; Extracellular matrix; Glycosaminoglycans; Heparan sulphate proteoglycan; Perivascular space; Perlecan.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All experimental and animal care procedures were in accordance with the Finnish and European legislation and were approved by the national Project Authorization Board (license numbers ESAVI/2362/04.10.07/2017 and ESAVI/13626/202121/2021). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Convective influx/glymphatic system: tracers injected into the CSF enter and leave the brain along separate periarterial basement membrane pathways.Acta Neuropathol. 2018 Jul;136(1):139-152. doi: 10.1007/s00401-018-1862-7. Epub 2018 May 12. Acta Neuropathol. 2018. PMID: 29754206 Free PMC article.
-
Border patrol: insights into the unique role of perlecan/heparan sulfate proteoglycan 2 at cell and tissue borders.Matrix Biol. 2014 Feb;34:64-79. doi: 10.1016/j.matbio.2013.08.004. Epub 2013 Aug 31. Matrix Biol. 2014. PMID: 24001398 Free PMC article. Review.
-
Heparan sulfate proteoglycan in diffuse plaques of hippocampus but not of cerebellum in Alzheimer's disease brain.Am J Pathol. 1994 Feb;144(2):337-47. Am J Pathol. 1994. PMID: 8311117 Free PMC article.
-
Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy.Acta Neuropathol. 2011 Apr;121(4):431-43. doi: 10.1007/s00401-011-0801-7. Epub 2011 Jan 23. Acta Neuropathol. 2011. PMID: 21259015
-
The role of brain barriers in fluid movement in the CNS: is there a 'glymphatic' system?Acta Neuropathol. 2018 Mar;135(3):387-407. doi: 10.1007/s00401-018-1812-4. Epub 2018 Feb 10. Acta Neuropathol. 2018. PMID: 29428972 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

