Pulmonary fibrosis: from mechanisms to therapies
- PMID: 40340941
- PMCID: PMC12063347
- DOI: 10.1186/s12967-025-06514-2
Pulmonary fibrosis: from mechanisms to therapies
Abstract
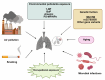
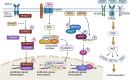
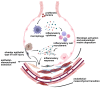
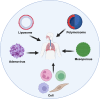
Pulmonary fibrosis (PF) is a chronic, progressive interstitial lung disease characterized by excessive deposition of extracellular matrix (ECM) and abnormal fibroblast proliferation, which is mainly caused by air pollution, smoking, aging, occupational exposure, environmental pollutants exposure, and microbial infections. Although antifibrotic agents such as pirfenidone and nintedanib, approved by the United States (US) Food and Drug Administration (FDA), can slow the decline in lung function and disease progression, their side effects and delivery inefficiency limit the overall prognosis of PF. Therefore, there is an urgent need to develop effective therapeutic targets and delivery approaches for PF in clinical settings. This review provides an overview of the pathogenic mechanisms, therapeutic drug targeting signaling pathways, and promising drug delivery strategies for treating PF.
Keywords: Drug delivery system; Pathogenic mechanisms; Pulmonary fibrosis; Therapeutic targets.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare that they have no known competing financial interests.
Figures
References
-
- Sun Y, Ren Y, Song LY, Wang YY, Li TG, Wu YL, Li L, Yang ZS. Targeting iron-metabolism:a potential therapeutic strategy for pulmonary fibrosis. Biomed Pharmacother. 2024;172:116270. - PubMed
-
- Fang Y, Chung SSW, Xu L, Xue C, Liu X, Jiang D, Li R, Korogi Y, Yuan K, Saqi A, Hibshoosh H, Huang Y, Lin CS, Tsukui T, Sheppard D, Sun X, J., Que. RUNX2 promotes fibrosis via an alveolar-to-pathological fibroblast transition, Nature, (2025). - PubMed
-
- Ren F, Aliper A, Chen J, Zhao H, Rao S, Kuppe C, Ozerov IV, Zhang M, Witte K, Kruse C, Aladinskiy V, Ivanenkov Y, Polykovskiy D, Fu Y, Babin E, Qiao J, Liang X, Mou Z, Wang H, Pun FW, Torres-Ayuso P, Veviorskiy A, Song D, Liu S, Zhang B, Naumov V, Ding X, Kukharenko A, Izumchenko E, Zhavoronkov A. A small-molecule TNIK inhibitor targets fibrosis in preclinical and clinical models. Nat Biotechnol. 2025;43:63–75. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- 82173554/National Natural Science Foundation of China
- 82203993/National Natural Science Foundation of China
- 82360641/National Natural Science Foundation of China
- 22KJB330003/The Natural Science Research of Jiangsu Higher Education Institutions
- 20232BAB206138/Jiangxi Provincial Natural Science Foundation
LinkOut - more resources
Full Text Sources
Medical

