Blocking LIF and PD-L1 enhances the antitumor efficacy of SBRT in murine PDAC models
- PMID: 40341024
- PMCID: PMC12067785
- DOI: 10.1136/jitc-2024-010820
Blocking LIF and PD-L1 enhances the antitumor efficacy of SBRT in murine PDAC models
Abstract
Background: Recent preclinical and clinical data suggest that leukemia inhibitory factor (LIF) is a potential target for various tumor types including pancreatic ductal adenocarcinoma as LIF is involved in multiple protumor processes including cancer stem cell maintenance, epithelial-mesenchymal transition (EMT), immunosuppression, and chemo/radioresistance. Anti-LIF antibody therapy has demonstrated safety and tolerability but limited efficacy in phase 1 clinical trial in advanced solid tumors. This prompted us to explore combination therapies, suggesting that LIF blockade, when combined with standard-of-care chemotherapy, radiotherapy, and/or immunotherapy, could present a promising therapeutic strategy.
Methods: We evaluated the impact of combining systemic inhibition of LIF/programmed death-ligand 1 (PD-L1) with localized stereotactic body radiotherapy (SBRT) on tumor progression across multiple murine orthotopic pancreatic tumor models and examined systemic antitumor immunity using a hepatic rechallenge model. The antitumor immune response was characterized throughflow cytometry and Luminex assays. To identify differentially expressed genes and signaling pathways following treatment, we performed bulk RNA sequencing on pancreatic tumors. Additionally, single-cell RNA sequencing was conducted to further examine changes in tumor-infiltrating immune cells and their signaling pathways.
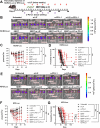
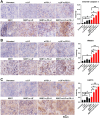
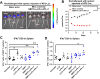
Results: We showed that simultaneous inhibition of LIF and PD-L1 significantly amplified the antitumor efficacy of SBRT, resulting in extended survival. The triple therapy (SBRT+anti-LIF+anti-PD-L1) generated an immunostimulatory tumor microenvironment, characterized by a proinflammatory shift in the cytokine/chemokine profile, increased infiltration of effector CD8+ T cells, and upregulated activation or maturation signals in tumor-infiltrating CD8+ T cells and macrophages. The beneficial effects of triple therapy were mostly abrogated by depletion of CD8+ T cells. In addition, triple therapy downregulated pathways related to tumor stemness, proliferation, and metabolism, and reduced EMT. Importantly, the combination of local SBRT treatment with systemic LIF and PD-L1 blockade resulted in long-term systemic antitumor memory.
Keywords: Gastrointestinal Cancer; Immunotherapy; Radiotherapy/radioimmunotherapy.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: JE and NL hold AstraZeneca stock.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
