The molecular mechanism and therapeutic landscape of copper and cuproptosis in cancer
- PMID: 40341098
- PMCID: PMC12062509
- DOI: 10.1038/s41392-025-02192-0
The molecular mechanism and therapeutic landscape of copper and cuproptosis in cancer
Abstract
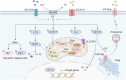
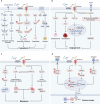
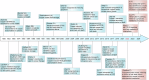
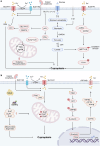
Copper, an essential micronutrient, plays significant roles in numerous biological functions. Recent studies have identified imbalances in copper homeostasis across various cancers, along with the emergence of cuproptosis, a novel copper-dependent form of cell death that is crucial for tumor suppression and therapeutic resistance. As a result, manipulating copper levels has garnered increasing interest as an innovative approach to cancer therapy. In this review, we first delineate copper homeostasis at both cellular and systemic levels, clarifying copper's protumorigenic and antitumorigenic functions in cancer. We then outline the key milestones and molecular mechanisms of cuproptosis, including both mitochondria-dependent and independent pathways. Next, we explore the roles of cuproptosis in cancer biology, as well as the interactions mediated by cuproptosis between cancer cells and the immune system. We also summarize emerging therapeutic opportunities targeting copper and discuss the clinical associations of cuproptosis-related genes. Finally, we examine potential biomarkers for cuproptosis and put forward the existing challenges and future prospects for leveraging cuproptosis in cancer therapy. Overall, this review enhances our understanding of the molecular mechanisms and therapeutic landscape of copper and cuproptosis in cancer, highlighting the potential of copper- or cuproptosis-based therapies for cancer treatment.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

