Myeloid but not hepatocytic CD38 is a key driver for hepatic ischemia/reperfusion injury
- PMID: 40341132
- PMCID: PMC12062225
- DOI: 10.1038/s41392-025-02233-8
Myeloid but not hepatocytic CD38 is a key driver for hepatic ischemia/reperfusion injury
Abstract
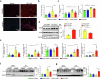
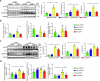
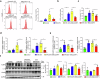
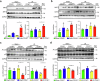
Hepatic ischemia-reperfusion injury (HIRI) is a critical condition that often occurs during liver transplantation and surgical liver resection. However, its mechanism has not been fully elucidated. Nicotinamide adenine dinucleotide (NAD+), functioning as a coenzyme or cofactor, is crucial for both redox and non-redox processes. In mammals, CD38 serves as the primary enzyme responsible for NAD+ degradation. In this study, we reported that the absence of CD38 markedly reduces HIRI in CD38 global knockout (CD38KO) and CD38 myeloid-specific knockout (CD38MKO) mice, but not in CD38 hepatocyte-specific knockout (CD38LKO) mice compared with the control (CD38fl/fl) mice by suppressing HIRI-induced hepatic oxidative stress, inflammatory responses, and pyroptosis. The findings were corroborated by a noticeable decrease in levels of alanine aminotransferase (ALT), aspartate transaminase (AST), and lactate dehydrogenase (LDH), along with reduced necrosis. Besides, we found that the expressions of SIRT1 and its downstream targets, p53 and PPARγ, were elevated in the liver tissues of CD38KO and CD38MKO mice compared to CD38fl/fl mice, while the acetylation levels of p53 were reduced. Furthermore, we demonstrated that myeloid CD38 deficiency not only promoted M2-type polarization and inhibited M1-type polarization of macrophages but also suppressed NLRP3-mediated pyroptosis by triggering NAD+/SIRT1 signaling in macrophages, resulting in the reduction of oxidative stress, inflammation, and pyroptosis in the liver, ultimately protecting against HIRI. This study highlights myeloid CD38 as a promising target for the prevention and treatment of HIRI clinically.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Myeloid Deletion of Cdc42 Protects Liver From Hepatic Ischemia-Reperfusion Injury via Inhibiting Macrophage-Mediated Inflammation in Mice.Cell Mol Gastroenterol Hepatol. 2024;17(6):965-981. doi: 10.1016/j.jcmgh.2024.01.023. Epub 2024 Feb 9. Cell Mol Gastroenterol Hepatol. 2024. PMID: 38342302 Free PMC article.
-
CD38 Deficiency Protects Mice from High Fat Diet-Induced Nonalcoholic Fatty Liver Disease through Activating NAD+/Sirtuins Signaling Pathways-Mediated Inhibition of Lipid Accumulation and Oxidative Stress in Hepatocytes.Int J Biol Sci. 2021 Oct 17;17(15):4305-4315. doi: 10.7150/ijbs.65588. eCollection 2021. Int J Biol Sci. 2021. PMID: 34803499 Free PMC article.
-
CD38 Deficiency Protects Mouse Retinal Ganglion Cells Through Activating the NAD+/Sirt1 Pathway in Ischemia-Reperfusion and Optic Nerve Crush Models.Invest Ophthalmol Vis Sci. 2024 May 1;65(5):36. doi: 10.1167/iovs.65.5.36. Invest Ophthalmol Vis Sci. 2024. PMID: 38776115 Free PMC article.
-
New progress in roles of nitric oxide during hepatic ischemia reperfusion injury.World J Gastroenterol. 2017 Apr 14;23(14):2505-2510. doi: 10.3748/wjg.v23.i14.2505. World J Gastroenterol. 2017. PMID: 28465634 Free PMC article. Review.
-
CD38 in Neurodegeneration and Neuroinflammation.Cells. 2020 Feb 18;9(2):471. doi: 10.3390/cells9020471. Cells. 2020. PMID: 32085567 Free PMC article. Review.
References
-
- Xiao, J. et al. Global liver disease burdens and research trends: Analysis from a Chinese perspective. J. Hepatol.71, 212–221 (2019). - PubMed
-
- Hirao, H., Nakamura, K. & Kupiec-Weglinski, J. W. Liver ischaemia-reperfusion injury: a new understanding of the role of innate immunity. Nat. Rev. Gastroenterol. Hepatol.19, 239–256 (2022). - PubMed
-
- Li, F. et al. The protective effect of PNU-282987, a selective α7 nicotinic acetylcholine receptor agonist, on the hepatic ischemia-reperfusion injury is associated with the inhibition of high-mobility group box 1 protein expression and nuclear factor κB activation in mice. Shock39, 197–203 (2013). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

