Structural and functional characterization of the SLA' structure at the 3' terminus of the Zika virus negative-strand intermediate
- PMID: 40341208
- PMCID: PMC12265937
- DOI: 10.1261/rna.080342.124
Structural and functional characterization of the SLA' structure at the 3' terminus of the Zika virus negative-strand intermediate
Abstract
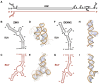
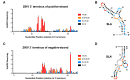
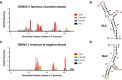
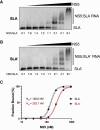
Flavivirus infections, including those of Dengue virus (DENV) and Zika virus (ZIKV), result in a high disease burden globally, yet many aspects of their viral life cycle remain poorly understood. For example, while some features of the mechanism of negative-strand RNA synthesis are known, relatively little is known about the initiation of positive-strand RNA synthesis in the flavivirus life cycle. Viral RNA replication is initiated via the recruitment of the viral NS5 RNA-dependent RNA polymerase (RdRp) to stem-loop A (SLA) at the 5' terminus of positive-strand genomic RNA. Subsequent genome cyclization is thought to facilitate loading of NS5 onto the 3' terminus of the genomic RNA to initiate negative-strand RNA synthesis. Conversely, it is not clear whether RNA structures in the negative-strand replicative intermediate similarly recruit NS5 to promote positive-strand RNA synthesis, providing specificity to this process. Herein, we characterized the secondary structure of the 3' terminus of the negative-strand replicative intermediate in ZIKV and DENV1-4 in silico and in vitro. We observed that the 3' terminus of the negative strand is capable of forming a secondary structure which mirrors SLA, which we term SLA'. While we demonstrate that SLA' forms in vitro and is capable of interacting with NS5, introduction of G·U wobble base pairs that disrupt SLA', while keeping SLA largely intact, suggest that SLA' is not necessary for viral RNA replication. As such, this work suggests that in contrast to related viruses, the positive-strand promoter is unlikely to be provided by specific structure(s) at the 3' terminus of the negative-strand replicative intermediate.
Keywords: SHAPE; Zika virus; flavivirus; negative-strand replicative intermediate; nonstructural protein 5 (NS5); stem–loop A (SLA).
© 2025 Abram et al.; Published by Cold Spring Harbor Laboratory Press for the RNA Society.
Figures


Similar articles
-
Interaction of West Nile virus NS5 with orthoflavivirus SLA RNAs and their effects on viral replication and inhibition.J Virol. 2025 Aug 18:e0202324. doi: 10.1128/jvi.02023-24. Online ahead of print. J Virol. 2025. PMID: 40824093
-
High-resolution RNA tertiary structures in Zika virus stem-loop A for the development of inhibitory small molecules.RNA. 2024 May 16;30(6):609-623. doi: 10.1261/rna.079796.123. RNA. 2024. PMID: 38383158 Free PMC article.
-
Cellular NONO protein binds to the flavivirus replication complex and promotes positive-strand RNA synthesis.J Virol. 2024 Dec 17;98(12):e0029724. doi: 10.1128/jvi.00297-24. Epub 2024 Nov 5. J Virol. 2024. PMID: 39499073 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Parents' and informal caregivers' views and experiences of communication about routine childhood vaccination: a synthesis of qualitative evidence.Cochrane Database Syst Rev. 2017 Feb 7;2(2):CD011787. doi: 10.1002/14651858.CD011787.pub2. Cochrane Database Syst Rev. 2017. PMID: 28169420 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
